Abstract
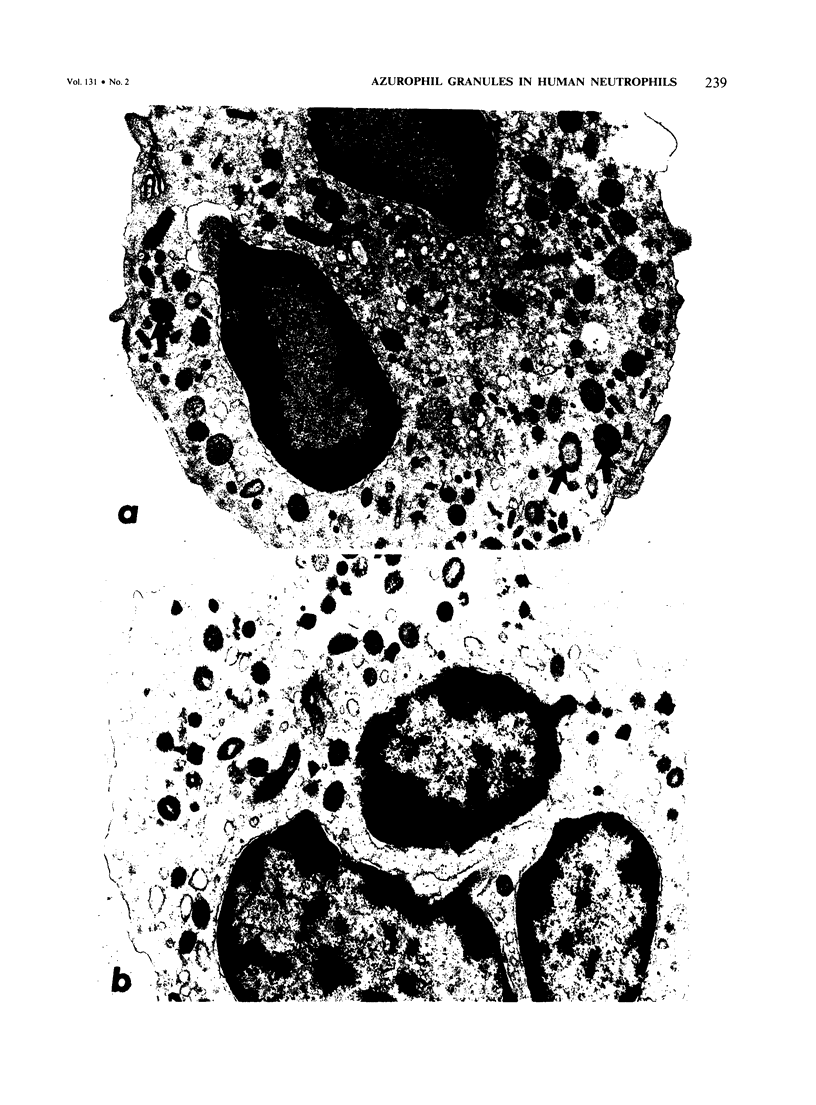
Previous ultrastructural studies of human neutrophils showed two distinctive granule types, the azurophil (peroxidase-positive) and the specific (peroxidase-negative). By identification of granules with peroxidase activity and those immunopositive for elastase antigen, the authors defined two subpopulations of azurophil granules, one that contained peroxidase activity and no measurable elastase antigen and another that contained elastase antigen associated with a small amount of peroxidase activity. They quantitated the peroxidase-positive as well as the elastase-positive granules in human peripheral blood neutrophils and found an average of 1536 +/- 69 peroxidase-positive granules per neutrophil. Of these, 399 +/- 20 were also elastase-positive. The average elastase concentration per neutrophil was 1.59 pg, and the average concentration per granule was 4 X 10(-3) pg. It is concluded that in normal individuals approximately one-third of the azurophil granules contain elastase antigen. Because neutrophil elastase has been implicated in the pathogenesis of emphysema, quantitation of its distribution within the cell presents an approach that may help define selective azurophil granule release and its relationship to the development of emphysema.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentwood B. J., Henson P. M. The sequential release of granule constitutents from human neutrophils. J Immunol. 1980 Feb;124(2):855–862. [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano V. V., Tsang A., Kucich U., Abrams W. R., Rosenbloom J., Kimbel P., Fallahnejad M., Weinbaum G. Immunolocalization of elastase in human emphysematous lungs. J Clin Invest. 1986 Aug;78(2):482–493. doi: 10.1172/JCI112600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Preventive therapy of emphysema: lessons from the elastase model. Am Rev Respir Dis. 1987 Apr;135(4):984–984. doi: 10.1164/arrd.1987.135.4.984a. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Kucich U., Abrams W. R., James H. L. Solid-phase immunoassay of dog neutrophil elastase. Anal Biochem. 1980 Dec;109(2):403–409. doi: 10.1016/0003-2697(80)90668-5. [DOI] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Association of lactoferrin with lysozyme in granules of human polymorphonuclear leukocytes. Infect Immun. 1972 Nov;6(5):761–765. doi: 10.1128/iai.6.5.761-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes J. F., Soares J. O., Alves de Matos A. P. Micro-buffy coats of whole blood: a method for the electron microscopic study of mononuclear cells. Stain Technol. 1979 Sep;54(5):257–260. doi: 10.3109/10520297909110681. [DOI] [PubMed] [Google Scholar]
- Parmley R. T., Takagi M., Barton J. C., Boxer L. A., Austin R. L. Ultrastructural localization of lactoferrin and iron-binding protein in human neutrophils and rabbit heterophils. Am J Pathol. 1982 Dec;109(3):343–358. [PMC free article] [PubMed] [Google Scholar]
- Perez H. D., Marder S., Elfman F., Ives H. E. Human neutrophils contain subpopulations of specific granules exhibiting different sensitivities to changes in cytosolic free calcium. Biochem Biophys Res Commun. 1987 Jun 15;145(2):976–981. doi: 10.1016/0006-291x(87)91061-8. [DOI] [PubMed] [Google Scholar]
- Pryzwansky K. B., Breton-Gorius J. Identification of a subpopulation of primary granules in human neutrophils based upon maturation and distribution. Study by transmission electron microscopy cytochemistry and high voltage electron microscopy of whole cell preparations. Lab Invest. 1985 Dec;53(6):664–671. [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- WEIBEL E. R., GOMEZ D. M. A principle for counting tissue structures on random sections. J Appl Physiol. 1962 Mar;17:343–348. doi: 10.1152/jappl.1962.17.2.343. [DOI] [PubMed] [Google Scholar]
- West B. C., Rosenthal A. S., Gelb N. A., Kimball H. R. Separation and characterization of human neutrophil granules. Am J Pathol. 1974 Oct;77(1):41–66. [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Damiano V. V., Tsang A. L., Meranze D. R., Glasgow J., Abrams W. R., Weinbaum G. Neurtrophil degranulation in cadmium-chloride-induced acute lung inflammation. Am J Pathol. 1982 Nov;109(2):145–156. [PMC free article] [PubMed] [Google Scholar]