Abstract
Cellular mechanisms of normal airway mucus secretion and their alterations in chronic obstructive lung disease are poorly understood. To aid in their study, the authors have produced a panel of monoclonal antibodies directed against various constituents of human airway secretions. Two fusions yielded 401 hybridoma-containing cultures. Supernatants from 150 of these cultures stained human tracheal secretory cells by immunofluorescence. Twenty-nine hybridomas were selected for expansion because they selectively stained a single cell type or displayed another interesting distribution. Antigens were further characterized by their localization in glycol methacrylate sections of human trachea, sensitivity to periodate oxidations, selective affinity for fraction peaks obtained by Sepharose 4B chromatography, and reactivity with molecules of various sizes, as estimated by SDS-PAGE. These antibodies will be useful for 1) quantitative detection of antigens in sputum or lavage samples by immunoassay and 2) purification and biochemical characterization of molecular constituents of airway secretions in health and disease.
Full text
PDF


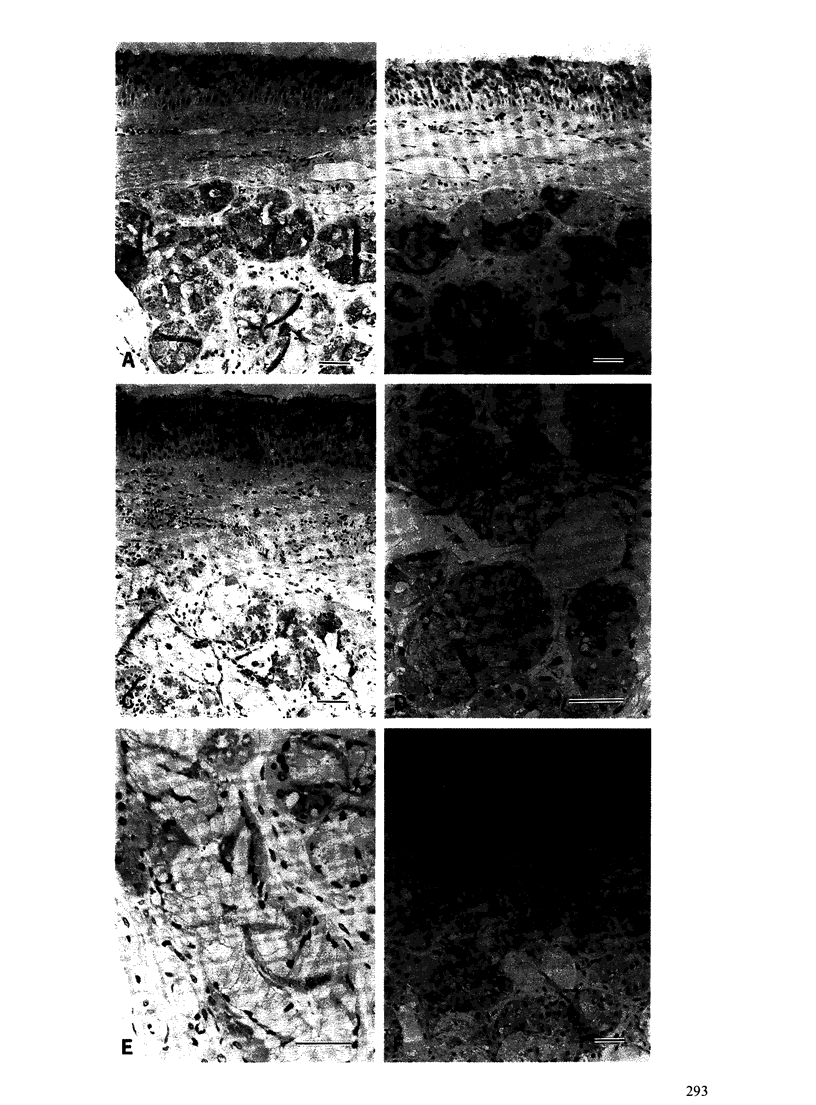
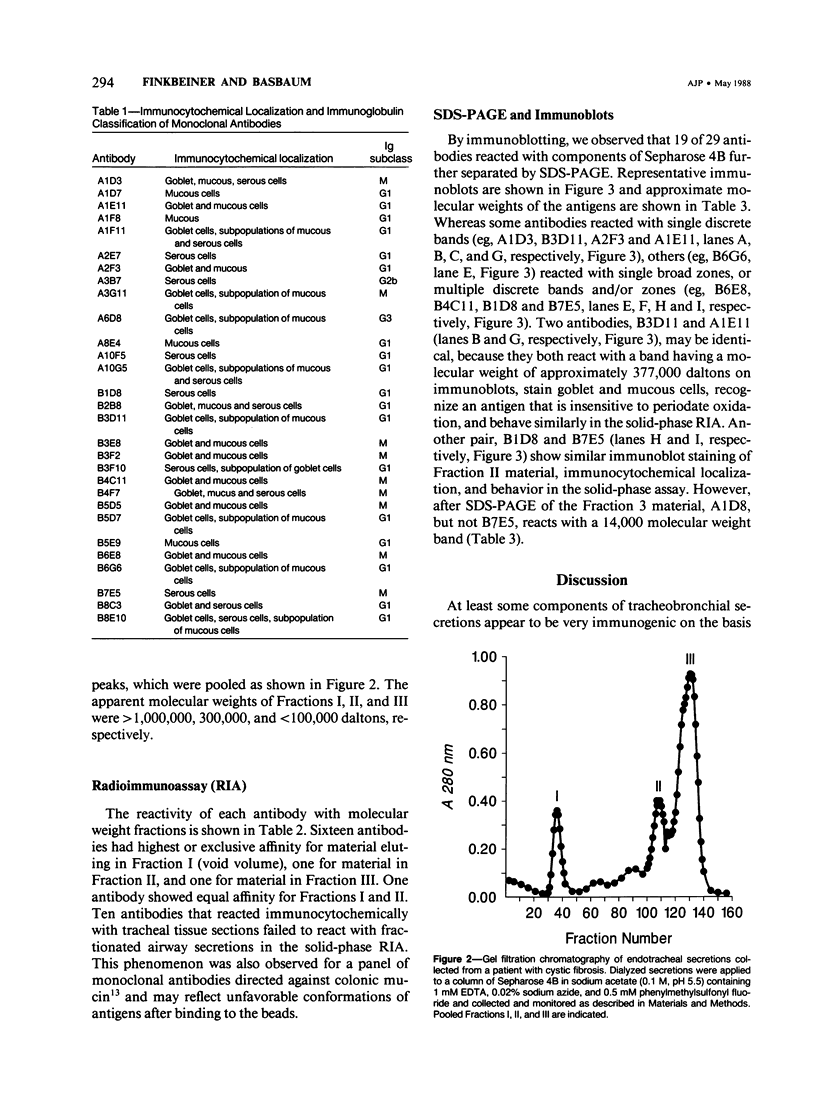
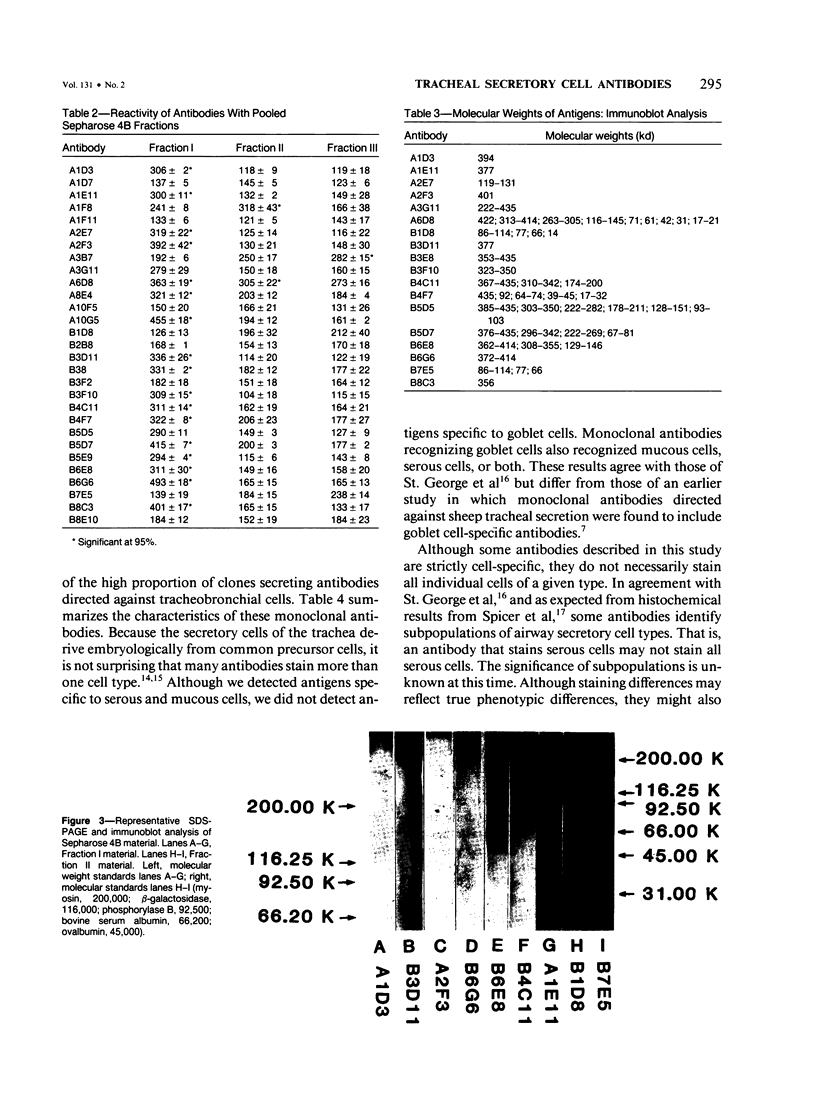
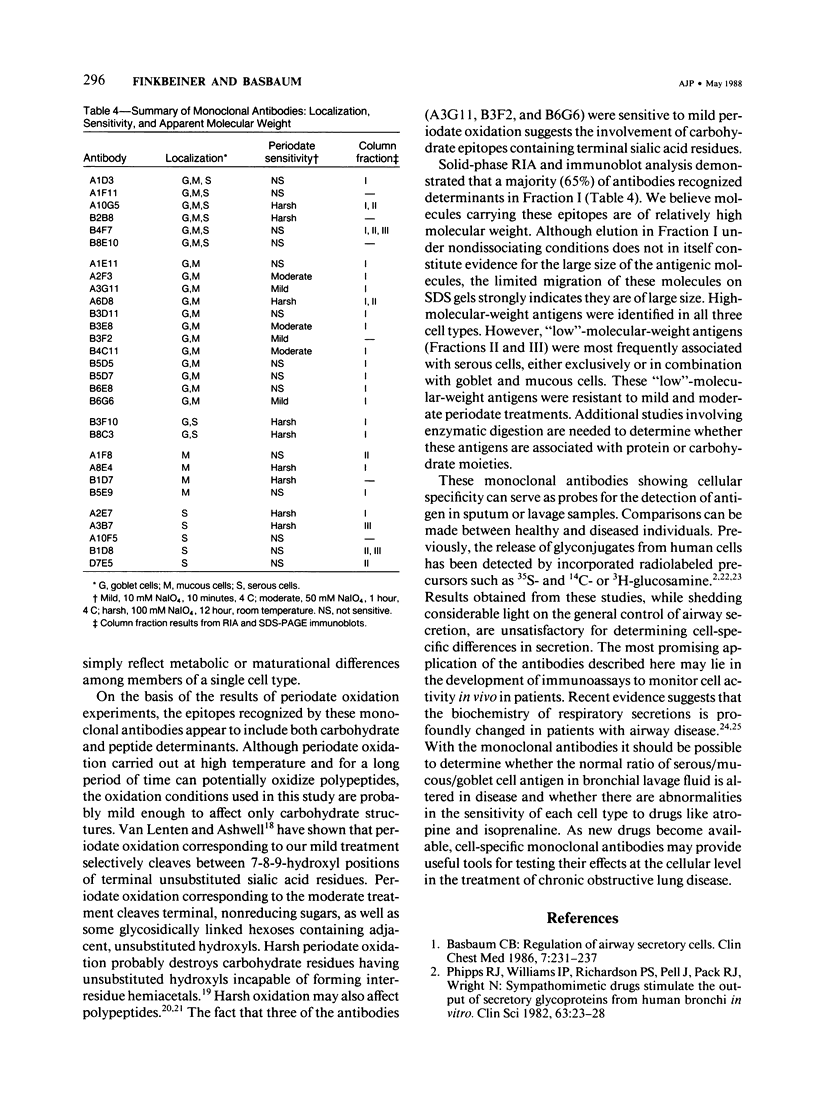

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basbaum C. B., Mann J. K., Chow A. W., Finkbeiner W. E. Monoclonal antibodies as probes for unique antigens in secretory cells of mixed exocrine organs. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4419–4423. doi: 10.1073/pnas.81.14.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum C. B. Regulation of airway secretory cells. Clin Chest Med. 1986 Jun;7(2):231–237. [PubMed] [Google Scholar]
- Beckstead J. H. Optimal antigen localization in human tissues using aldehyde-fixed plastic-embedded sections. J Histochem Cytochem. 1985 Sep;33(9):954–958. doi: 10.1177/33.9.4020104. [DOI] [PubMed] [Google Scholar]
- Bhaskar K. R., O'Sullivan D. D., Opaskar-Hincman H., Reid L. M., Coles S. J. Density gradient analysis of secretions produced in vitro by human and canine airway mucosa: identification of lipids and proteoglycans in such secretions. Exp Lung Res. 1986;10(4):401–422. doi: 10.3109/01902148609058290. [DOI] [PubMed] [Google Scholar]
- Bhaskar K. R., O'Sullivan D. D., Seltzer J., Rossing T. H., Drazen J. M., Reid L. M. Density gradient study of bronchial mucus aspirates from healthy volunteers (smokers and nonsmokers) and from patients with tracheostomy. Exp Lung Res. 1985;9(3-4):289–308. doi: 10.3109/01902148509057529. [DOI] [PubMed] [Google Scholar]
- CLAMP J. R., HOUGH L. THE PERIODATE OXIDATION OF AMINO ACIDS WITH REFERENCE TO STUDIES ON GLYCOPROTEINS. Biochem J. 1965 Jan;94:17–24. doi: 10.1042/bj0940017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles S. J., Reid L. Inhibition of glycoconjugate secretion by colchicine and cytochalasin B. An in vitro study of human airway. Cell Tissue Res. 1981;214(1):107–118. doi: 10.1007/BF00235149. [DOI] [PubMed] [Google Scholar]
- Krotoski W. A., Weimer H. E. Peptide-associated and antigenic changes accompanying periodic acid oxidation of human plasma orosomucoid. Arch Biochem Biophys. 1966 Aug;115(2):337–344. doi: 10.1016/0003-9861(66)90284-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marom Z., Shelhamer J. H., Kaliner M. Effects of arachidonic acid, monohydroxyeicosatetraenoic acid and prostaglandins on the release of mucous glycoproteins from human airways in vitro. J Clin Invest. 1981 Jun;67(6):1695–1702. doi: 10.1172/JCI110207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps R. J., Williams I. P., Richardson P. S., Pell J., Pack R. J., Wright N. Sympathomimetic drugs stimulate the output of secretory glycoproteins from human bronchi in vitro. Clin Sci (Lond) 1982 Jul;63(1):23–28. doi: 10.1042/cs0630023. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A., Lynch K. E. Development of anti-human colonic mucin monoclonal antibodies. Characterization of multiple colonic mucin species. J Clin Invest. 1986 Apr;77(4):1251–1262. doi: 10.1172/JCI112428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A., Lynch K. E. Human colonic goblet cells. Demonstration of distinct subpopulations defined by mucin-specific monoclonal antibodies. J Clin Invest. 1986 Apr;77(4):1263–1271. doi: 10.1172/JCI112429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelhamer J. H., Marom Z., Kaliner M. Immunologic and neuropharmacologic stimulation of mucous glycoprotein release from human airways in vitro. J Clin Invest. 1980 Dec;66(6):1400–1408. doi: 10.1172/JCI109993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer S. S., Chakrin L. W., Wardell J. R., Jr, Kendrick W. Histochemistry of mucosubstances in the canine and human respiratory tract. Lab Invest. 1971 Dec;25(6):483–490. [PubMed] [Google Scholar]
- St George J. A., Cranz D. L., Zicker S. C., Etchison J. R., Dungworth D. L., Plopper C. G. An immunohistochemical characterization of rhesus monkey respiratory secretions using monoclonal antibodies. Am Rev Respir Dis. 1985 Sep;132(3):556–563. doi: 10.1164/arrd.1985.132.3.556. [DOI] [PubMed] [Google Scholar]
- THURLBECK W. M., BENJAMIN B., REID L. A sampling method for estimating the number of mucous glands in the foetal human trachea. Br J Dis Chest. 1961 Apr;55:49–53. doi: 10.1016/s0007-0971(61)80001-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971 Mar 25;246(6):1889–1894. [PubMed] [Google Scholar]
- Werdelin O., Ranlov P. Amyloidosis in mice produced by transplantation of spleen cells from casein-treated mice. Acta Pathol Microbiol Scand. 1966;68(1):1–18. doi: 10.1111/apm.1966.68.1.1. [DOI] [PubMed] [Google Scholar]




