Abstract
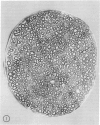
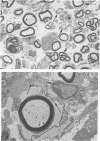
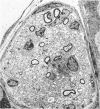
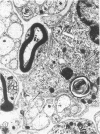
The twitcher is an authentic murine model of globoid cell leukodystrophy (GLD) in man. Extensive demyelination of the central and peripheral nervous systems (CNS and PNS) characterizes the neuropathologic features of GLD. In the common peroneal nerve of the twitcher, where demyelination was extensive, pronounced morphologic and quantitative alterations were noted in the unmyelinated fibers. They were 1) a large number of long and attenuated cellular processes of Schwann cells, which often enclosed only one or two axons; and 2) a threefold increase in the number of Schwann cell-axon units with reduced numbers of axons per unit. These results suggested increased branching of unmyelinated Schwann cells. Mild increase in unmyelinated fibers and mild decrease in myelinated fibers were additional features. In contrast, the sympathetic nerve trunk, which had only small numbers of myelinated and rare or no demyelinated fibers, showed much milder alterations in the unmyelinated fibers. Thus, the results of our study suggest that the alterations of the Schwann cells of the unmyelinated fibers in the twitcher are secondary to or in association with the chronic demyelinating process.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray G. M., Aguayo A. J., Martin J. B. Immunosympathectomy: late effects on the composition of rat cervical sympathetic trunks and influence on axonal regeneration after crush. Acta Neuropathol. 1973 Dec 3;26(4):345–352. doi: 10.1007/BF00688081. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Eicher E. M., Jacobs J. M., Scaravilli F., Teixeira F. Hereditary leucodystrophy in the mouse: the new mutant twitcher. Brain. 1980 Sep;103(3):695–710. doi: 10.1093/brain/103.3.695. [DOI] [PubMed] [Google Scholar]
- Fields K. L., Yen S. H. A subset of Schwann cells in peripheral nerves contain a 50-kDa protein antigenically related to astrocyte intermediate filaments. J Neuroimmunol. 1985 Jun;8(4-6):311–330. doi: 10.1016/s0165-5728(85)80070-9. [DOI] [PubMed] [Google Scholar]
- Jacobs J. M., Scaravilli F., De Aranda F. T. The pathogenesis of globoid cell leucodystrophy in peripheral nerve of the mouse mutant twitcher. J Neurol Sci. 1982 Sep;55(3):285–304. doi: 10.1016/0022-510x(82)90127-7. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R. Glial fibrillary acidic polypeptides in peripheral glia. Molecular weight, heterogeneity and distribution. J Neuroimmunol. 1985 Jun;8(4-6):377–393. doi: 10.1016/s0165-5728(85)80074-6. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R. Nonmyelin-forming Schwann cells coexpress surface proteins and intermediate filaments not found in myelin-forming cells: a study of Ran-2, A5E3 antigen and glial fibrillary acidic protein. J Neurocytol. 1984 Dec;13(6):923–934. doi: 10.1007/BF01148594. [DOI] [PubMed] [Google Scholar]
- Joosten E. M., Krijgsman J. B., Gabreëls-Festen A. A., Gabreëels F. J., Baars P. E. Infantile globoid cell leucodystrophy (Krabbe's disease). Some remarks on clinical, biochemical and sural nerve biopsy findings. Neuropadiatrie. 1974 May;5(2):191–209. doi: 10.1055/s-0028-1091702. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Chiu F. C., Katayama M., Sacchi R. S., Suzuki K., Suzuki K. Expression of glial fibrillary acidic protein in the CNS and PNS of murine globoid cell leukodystrophy, the twitcher. Am J Pathol. 1986 Nov;125(2):227–243. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Nagara H., Suzuki K., Suzuki K. The twitcher mouse: determination of genetic status by galactosylceramidase assays on clipped tail. Biochem Med. 1982 Feb;27(1):8–14. doi: 10.1016/0006-2944(82)90003-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Yamanaka T., Jacobs J. M., Teixeira F., Suzuki K. The Twitcher mouse: an enzymatically authentic model of human globoid cell leukodystrophy (Krabbe disease). Brain Res. 1980 Dec 8;202(2):479–483. doi: 10.1016/0006-8993(80)90159-6. [DOI] [PubMed] [Google Scholar]
- Martin J. J., Ceuterick C., Martin L., Leroy J. G., Nuyts J. P., Joris C. Leucodystrophie à cellules globoïdes (maladie de Krabbe). Lésions nerveuses périphériques. Acta Neurol Belg. 1974 Nov-Dec;74(6):356–375. [PubMed] [Google Scholar]
- Ochoa J., Mair W. G. The normal sural nerve in man. II. Changes in the axons and Schwann cells due to ageing. Acta Neuropathol. 1969;13(3):217–239. doi: 10.1007/BF00690643. [DOI] [PubMed] [Google Scholar]
- Ochoa J. Recognition of unmyelinated fiber disease: morphologic criteria. Muscle Nerve. 1978 Sep-Oct;1(5):375–387. doi: 10.1002/mus.880010506. [DOI] [PubMed] [Google Scholar]
- Ochoa J., Vial J. D. Behaviour of peripheral nerve structures in chronic neuropathies, with special reference to the Schwann cell. J Anat. 1967 Nov;102(Pt 1):95–111. [PMC free article] [PubMed] [Google Scholar]
- Powell H. C., Knobler R. L., Myers R. R. Peripheral neuropathy in the Twitcher mutant. A new experimental model of endoneurial edema. Lab Invest. 1983 Jul;49(1):19–25. [PubMed] [Google Scholar]
- Schlaepfer W. W., Prensky A. L. Quantitative and qualitative study of sural nerve biopsies in Krabbe's disease. Acta Neuropathol. 1972;20(1):55–66. doi: 10.1007/BF00687902. [DOI] [PubMed] [Google Scholar]
- Thomas P. K., King R. H. The degeneration of unmyelinated axons following nerve section: an ultrastructural study. J Neurocytol. 1974 Oct;3(4):497–512. doi: 10.1007/BF01098736. [DOI] [PubMed] [Google Scholar]
- Yen S. H., Fields K. L. Antibodies to neurofilament, glial filament, and fibroblast intermediate filament proteins bind to different cell types of the nervous system. J Cell Biol. 1981 Jan;88(1):115–126. doi: 10.1083/jcb.88.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]