Abstract
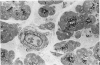
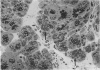
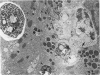
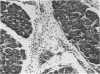
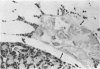
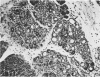
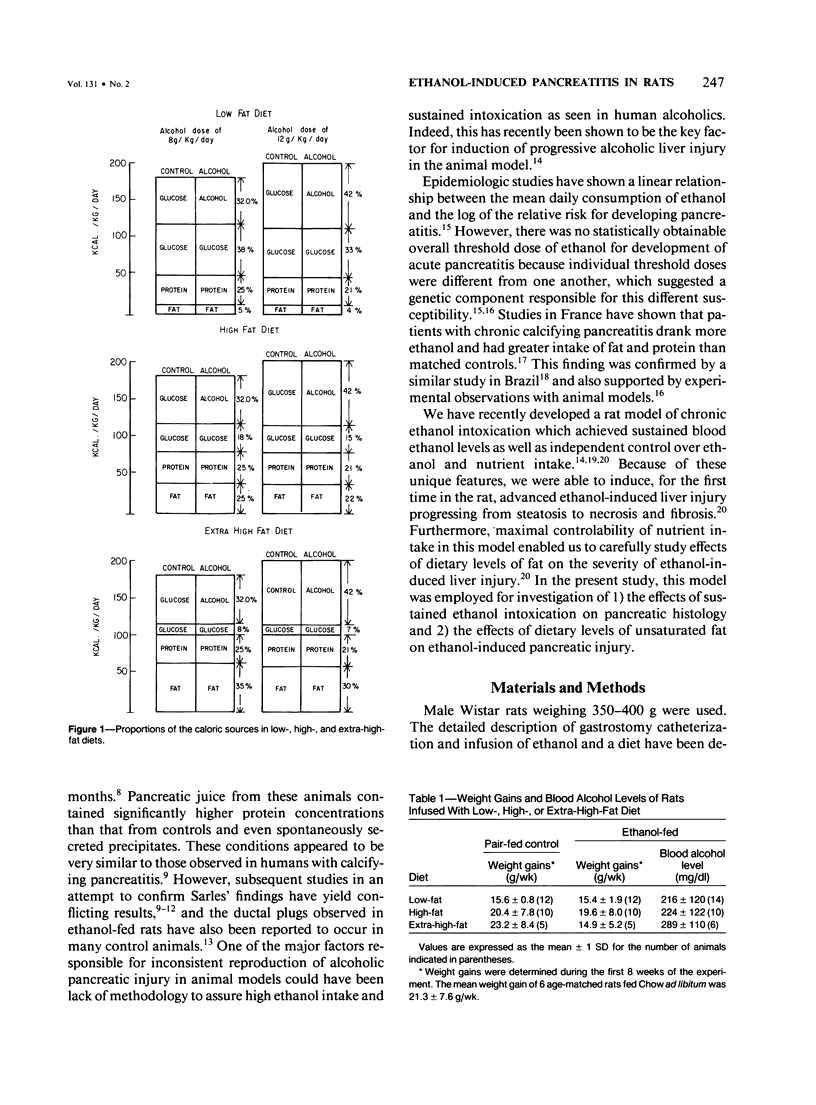
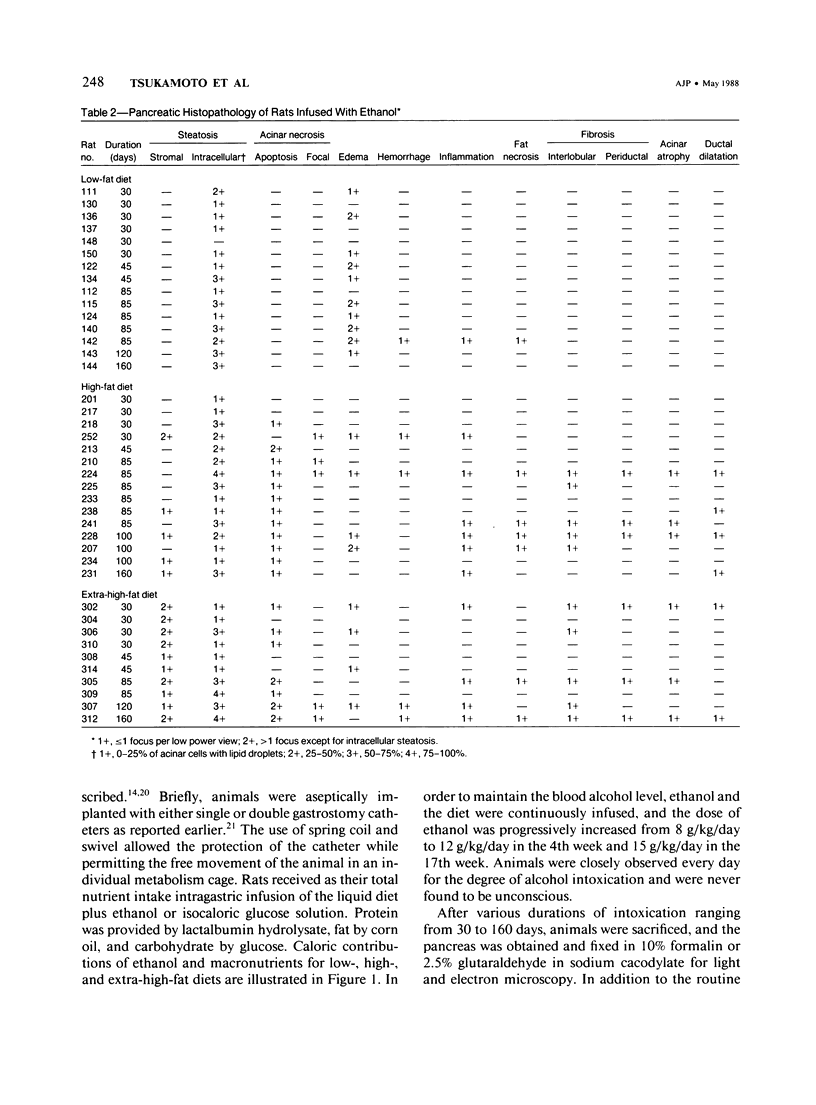
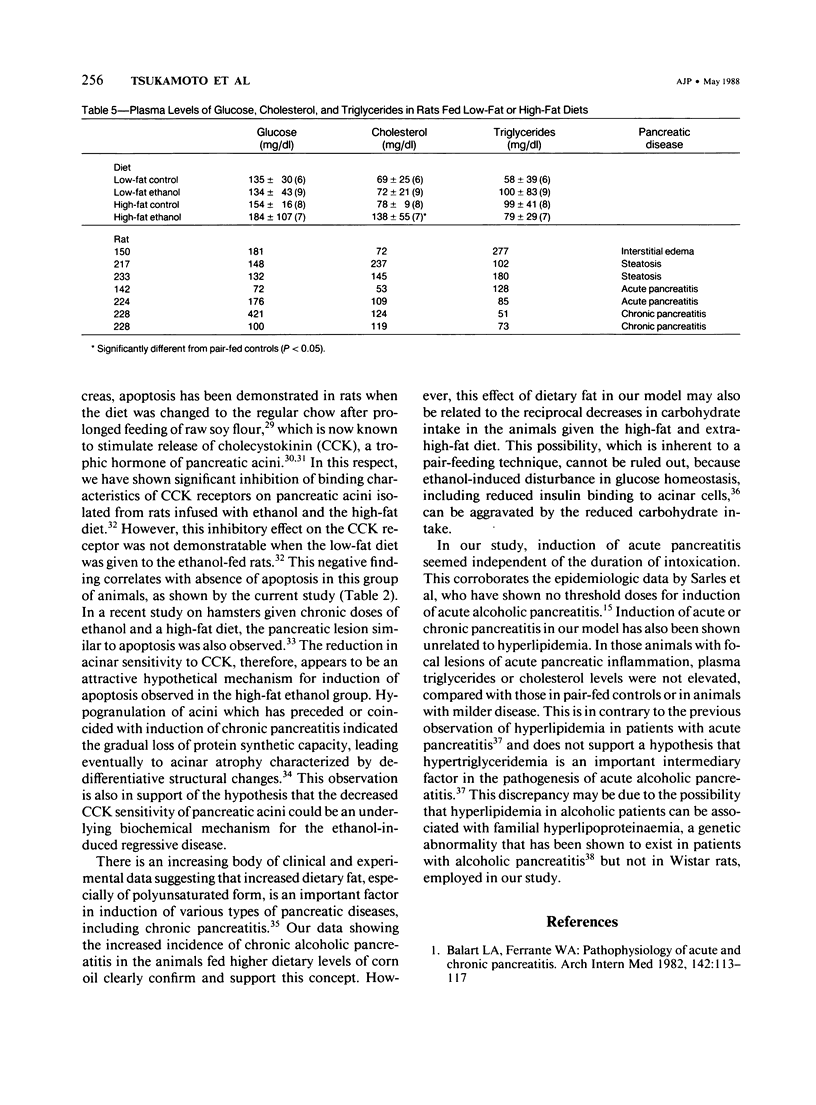
Effects of sustained ethanol intoxication and dietary fat content on pancreatic morphology were investigated in the rat model implanted with gastrostomy catheters, which permitted continuous intragastric infusion of ethanol plus liquid diet containing one of three levels of corn oil: 5% (low-fat), 25% (high-fat), and 35% (extra-high-fat) of total calories. After various durations of infusion ranging from 30 to 160 days, the pancreatic histology was examined. Mean blood alcohol levels achieved in the low, high, and extra-high fat diet groups were similarly high: 210 +/- 120, 224 +/- 122, and 289 +/- 110 mg/dl. The average weight gain of these ethanol-fed groups during the first 8 weeks of experiments was 15.4 +/- 1.9, 19.6 +/- 8.0, and 14.9 +/- 5.2 g/wk, respectively, and was not statistically different from that of pair-fed controls infused with isocaloric amount of dextrose and respective diet, nor from that of age-matched animals given the regular chow. None of control animals showed abnormal pancreatic morphologic features except occasional mild steatosis in those fed the extra-high-fat diet. With the low dietary intake of unsaturated fat, chronic ethanol intoxication produced only mild pancreatic pathology such as steatosis and interstitial edema. Administration of ethanol and the high-fat and extra-high-fat diets caused hypogranulation and apoptosis of acinar cells. Focal lesions of chronic pancreatitis were also observed in 20% or 30% of ethanol-fed animals given the high-fat or extra-high-fat diet. These lesions were characterized by fat necrosis, mononuclear cell infiltration, fibrosis, acinar atrophy, ductal dilatation, and intraductal mucious or proteinacious plugs. The incidence of focal acute pancreatitis was less (7-20%) but appeared increased with higher dietary fat content. Induction of either acute or chronic pancreatitis was not correlated with plasma levels of triglycerides or cholesterol. These results demonstrate potentiation by dietary unsaturated fat of ethanol-induced pancreatic injury. This model possesses many features analogous to those seen in alcoholic pancreatic injury in man. The hyperlipidemia does not appear to be an important pathogenetic factor for ethanol-induced pancreatitis produced in this model.
Full text
PDF


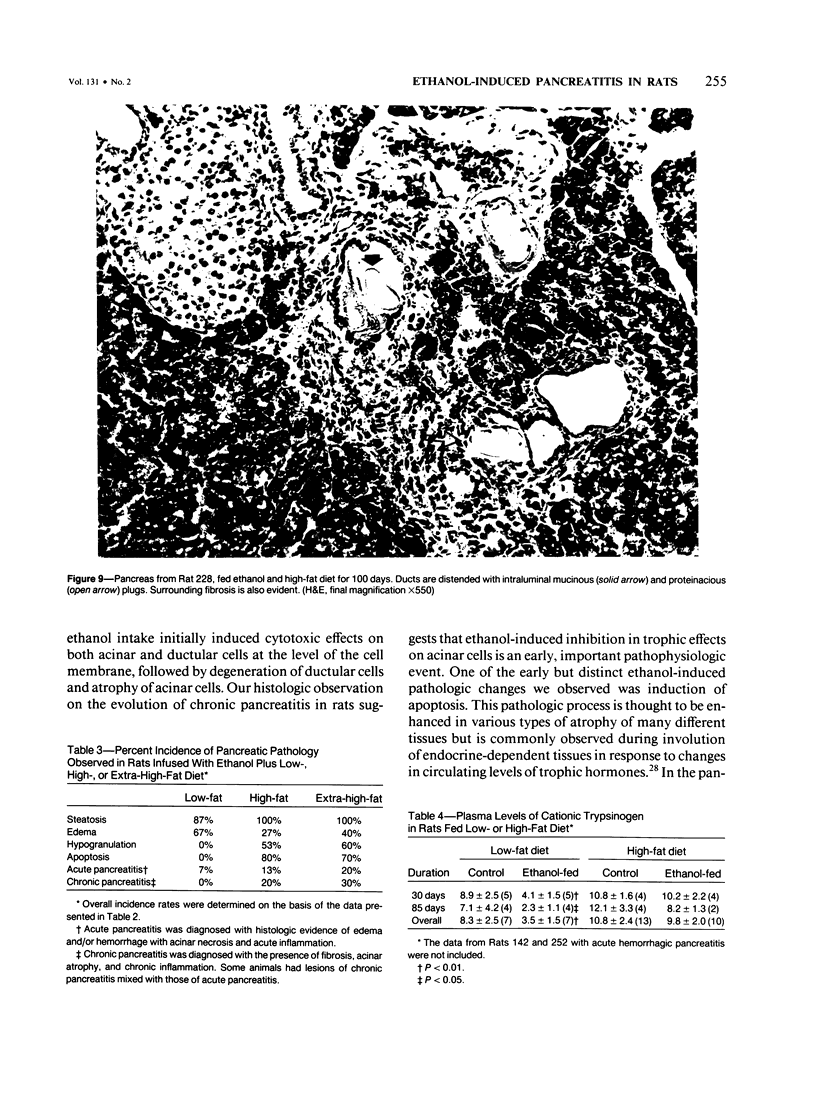

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balart L. A., Ferrante W. A. Pathophysiology of acute and chronic pancreatitis. Arch Intern Med. 1982 Jan;142(1):113–117. [PubMed] [Google Scholar]
- Bockman D. E., Singh M., Laugier R., Sarles H. Alcohol and the integrity of the pancreas. Scand J Gastroenterol Suppl. 1985;112:106–113. doi: 10.3109/00365528509092220. [DOI] [PubMed] [Google Scholar]
- Bordalo O., Goncalves D., Noronha M., Cristina M. L., Salgadinho A., Dreiling D. A. Newer concept for the pathogenesis of chronic alcoholic pancreatitis. Am J Gastroenterol. 1977;68(3):278–285. [PubMed] [Google Scholar]
- Braganza J. M. Pancreatic disease: a casualty of hepatic "detoxification"? Lancet. 1983 Oct 29;2(8357):1000–1003. doi: 10.1016/s0140-6736(83)90983-2. [DOI] [PubMed] [Google Scholar]
- Cameron J. L., Zuidema G. D., Margolis S. A pathogenesis for alcoholic pancreatitis. Surgery. 1975 Jun;77(6):754–763. [PubMed] [Google Scholar]
- Crass R. A., Morgan R. G. Rapid changes in pancreatic DNA, RNA and protein in the rat during pancreatic enlargement and involution. Int J Vitam Nutr Res. 1981;51(1):85–91. [PubMed] [Google Scholar]
- Darle N., Ekholm R., Edlund Y. Ultrastructure of the rat exocrine pancreas after long term intake of ethanol. Gastroenterology. 1970 Jan;58(1):62–72. [PubMed] [Google Scholar]
- Dickson A. P., O'Neill J., Imrie C. W. Hyperlipidaemia, alcohol abuse and acute pancreatitis. Br J Surg. 1984 Sep;71(9):685–688. doi: 10.1002/bjs.1800710913. [DOI] [PubMed] [Google Scholar]
- Durbec J. P., Sarles H. Multicenter survey of the etiology of pancreatic diseases. Relationship between the relative risk of developing chronic pancreaitis and alcohol, protein and lipid consumption. Digestion. 1978;18(5-6):337–350. doi: 10.1159/000198221. [DOI] [PubMed] [Google Scholar]
- Guy O., Robles-Diaz G., Adrich Z., Sahel J., Sarles H. Protein content of precipitates present in pancreatic juice of alcoholic subjects and patients with chronic calcifying pancreatitis. Gastroenterology. 1983 Jan;84(1):102–107. [PubMed] [Google Scholar]
- Howes R., Zuidema G. D., Cameron J. L. Evaluation of prophylactic antibiotics in acute pancreatitis. J Surg Res. 1975 Feb;18(2):197–200. doi: 10.1016/0022-4804(75)90016-5. [DOI] [PubMed] [Google Scholar]
- Hubens A., De Schepper A. Hepatic artery aneurysm: a pitfall in biliary surgery. Br J Surg. 1979 Apr;66(4):259–261. doi: 10.1002/bjs.1800660413. [DOI] [PubMed] [Google Scholar]
- Jordan G. L., Jr, Spjut H. J. Hemorrhagic pancreatitis. Arch Surg. 1972 Apr;104(4):489–493. doi: 10.1001/archsurg.1972.04180040103018. [DOI] [PubMed] [Google Scholar]
- Kagaya T., Takebe T., Koizumi M., Kataoka S., Kamei T., Oyama K. Effect of long term alcohol feeding on the pancreas in rat. Gastroenterol Jpn. 1979 Aug;14(4):327–335. doi: 10.1007/BF02774229. [DOI] [PubMed] [Google Scholar]
- Kakizaki G., Sasahara M., Aikawa T., Matsuo M., Sugawara Y., Nakamura K., Endo S., Ito Y. On the pathogenesis of chronic alcoholic pancreatitis from the viewpoint of experimental results in rats. Int J Pancreatol. 1987 Apr;2(2):101–116. doi: 10.1007/BF03015003. [DOI] [PubMed] [Google Scholar]
- Logsdon C. D. Stimulation of pancreatic acinar cell growth by CCK, epidermal growth factor, and insulin in vitro. Am J Physiol. 1986 Oct;251(4 Pt 1):G487–G494. doi: 10.1152/ajpgi.1986.251.4.G487. [DOI] [PubMed] [Google Scholar]
- Lundh G. Pankreatit--nya synpunkter på etiologi, diagnostik och behandling. Nord Med. 1970 Oct 22;84(43):1353–1359. [PubMed] [Google Scholar]
- Matsuno S., Kano K., Miyagawa K., Yamauchi H., Sato T. Effects of long term intravenous administration of ethanol on rat pancreas. Tohoku J Exp Med. 1983 Sep;141(1):77–89. doi: 10.1620/tjem.141.77. [DOI] [PubMed] [Google Scholar]
- Papp M., Fodor I., Varga G. Development of intraductal protein plugs in rats fed with ethanol for 18 months. Acta Morphol Hung. 1984;32(1):31–35. [PubMed] [Google Scholar]
- Sarles H. An international survey on nutrition and pancreatitis. Digestion. 1973;9(5):389–403. doi: 10.1159/000197468. [DOI] [PubMed] [Google Scholar]
- Sarles H. Chronic calcifying pancreatitis--chronic alcoholic pancreatitis. Gastroenterology. 1974 Apr;66(4):604–616. [PubMed] [Google Scholar]
- Sarles H., Lebreuil G., Tasso F., Figarella C., Clemente F., Devaux M. A., Fagonde B., Payan H. A comparison of alcoholic pancreatitis in rat and man. Gut. 1971 May;12(5):377–388. doi: 10.1136/gut.12.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarles H., Sarles J. C., Camatte R., Muratore R., Gaini M., Guien C., Pastor J., Le Roy F. Observations on 205 confirmed cases of acute pancreatitis, recurring pancreatitis, and chronic pancreatitis. Gut. 1965 Dec;6(6):545–559. doi: 10.1136/gut.6.6.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., LaSure M. M., Bockman D. E. Pancreatic acinar cell function and morphology in rats chronically fed an ethanol diet. Gastroenterology. 1982 Mar;82(3):425–434. [PubMed] [Google Scholar]
- Temler R. S., Dormond C. A., Simon E., Morel B. The effect of feeding soybean trypsin inhibitor and repeated injections of cholecystokinin on rat pancreas. J Nutr. 1984 Jun;114(6):1083–1091. doi: 10.1093/jn/114.6.1083. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H., Delgado G., Reidelberger R. D., Largman C. Effects of cholecystokinin, food intake and cephalic stimuli on plasma levels of amylase, lipase, and immunoreactive cationic trypsinogen in rats. Digestion. 1986;35(2):69–77. doi: 10.1159/000199349. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H., French S. W., Benson N., Delgado G., Rao G. A., Larkin E. C., Largman C. Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology. 1985 Mar-Apr;5(2):224–232. doi: 10.1002/hep.1840050212. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H., French S. W., Reidelberger R. D., Largman C. Cyclical pattern of blood alcohol levels during continuous intragastric ethanol infusion in rats. Alcohol Clin Exp Res. 1985 Jan-Feb;9(1):31–37. doi: 10.1111/j.1530-0277.1985.tb05046.x. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H., Reidelberger R. D., French S. W., Largman C. Long-term cannulation model for blood sampling and intragastric infusion in the rat. Am J Physiol. 1984 Sep;247(3 Pt 2):R595–R599. doi: 10.1152/ajpregu.1984.247.3.R595. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H., Sankaran H., Delgado G., Reidelberger R. D., Deveney C. W., Largman C. Increased pancreatic acinar content and secretion of cationic trypsinogen following 30-day continuous ethanol intoxication in rats. Biochem Pharmacol. 1986 Oct 15;35(20):3623–3629. doi: 10.1016/0006-2952(86)90635-0. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H., Towner S. J., Ciofalo L. M., French S. W. Ethanol-induced liver fibrosis in rats fed high fat diet. Hepatology. 1986 Sep-Oct;6(5):814–822. doi: 10.1002/hep.1840060503. [DOI] [PubMed] [Google Scholar]
- Uys C. J., Bank S., Marks I. N. The pathology of chronic pancreatitis in Cape Town. Digestion. 1973;9(5):454–468. doi: 10.1159/000197474. [DOI] [PubMed] [Google Scholar]
- Weesner R. E., Ruffolo J. J., Murphy R. F., Dincsoy H. P., Mendenhall C. L. Effect of chronic ethanol consumption on the pancreas of the hamster. Dig Dis Sci. 1985 Feb;30(2):168–177. doi: 10.1007/BF01308205. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]