Abstract
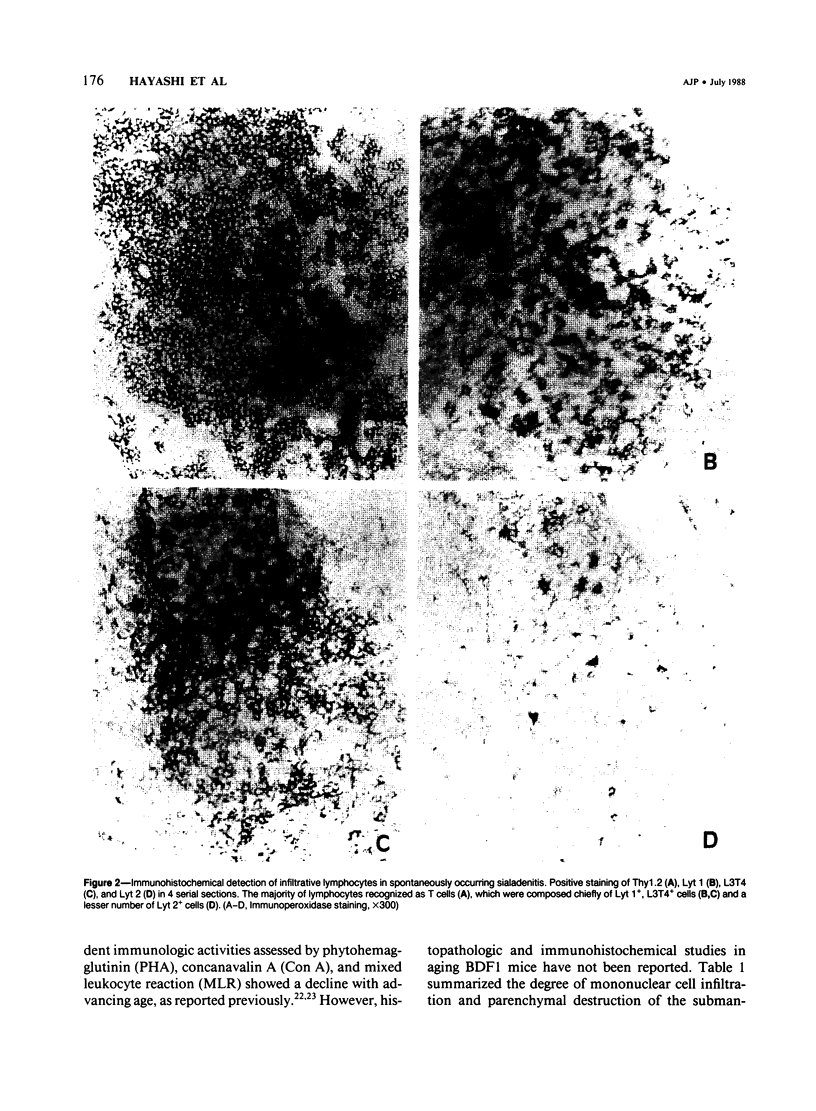
This study reports that spontaneous autoimmune sialadenitis developed in aging female, rather than male, BDF1 mice. The lesions first appeared in 6-month-old female BDF1 mice and were aggravated with advancing age, especially in 24-month-old and 30-month-old senescent mice. In contrast, significant inflammatory changes did not develop in aging male BDF1 mice. The presence of antisalivary duct antibody was found in sera from mice with sialadenitis. The infiltrating cells in the lesions of submandibular salivary glands were mainly composed of T cells, especially Lyt 1+ and L3T4+ cells. Moreover, mild inflammatory lesions were observed in parotid, sublingual salivary glands, pancreas, or kidneys in some mice that developed spontaneously occurring sialadenitis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson T. C., 3rd, Fox R. I., Frisman D. M., Howell F. V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983 Jan;130(1):203–208. [PubMed] [Google Scholar]
- Bullock J. Y., Booth R. J., Wilson J. D. Tissue antibodies in a healthy New Zealand population. N Z Med J. 1979 Jan 10;89(627):11–13. [PubMed] [Google Scholar]
- Carlsö B., Ostberg Y. Ultrastructural observations on the parotitis autoimmunica in the NZB/NZW hybrid mice. Acta Otolaryngol. 1978 Mar-Apr;85(3-4):298–306. doi: 10.3109/00016487809111939. [DOI] [PubMed] [Google Scholar]
- Greenspan J. S., Daniels T. E., Talal N., Sylvester R. A. The histopathology of Sjögren's syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974 Feb;37(2):217–229. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- Greenspan J. S., Gutman G. A., Weissman I. L., Talal N. Thymus-antigen- and immunoglobulin-positive lymphocytes in tissue infiltrates of NZB/NZW mice. Clin Immunol Immunopathol. 1974 Sep;3(1):16–31. doi: 10.1016/0090-1229(74)90020-8. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Sato M., Hirokawa K. Induction of experimental allergic sialadenitis in mice. Am J Pathol. 1985 Mar;118(3):476–483. [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Yura Y., Yoshida H., Yanagawa T., Sato M. Development of allergic sialadenitis in mice immunized with mumps virus-infected submandibular salivary gland. Am J Pathol. 1986 May;123(2):271–279. [PMC free article] [PubMed] [Google Scholar]
- Hirokawa K. Autoimmunity and aging. Concepts Immunopathol. 1985;1:251–288. [PubMed] [Google Scholar]
- Hirokawa K., Utsuyama M., Goto H., Kuramoto K. Differential rate of age-related decline in immune functions in genetically defined mice with different tumor incidence and life span. Gerontology. 1984;30(4):223–233. doi: 10.1159/000212636. [DOI] [PubMed] [Google Scholar]
- Jabs D. A., Prendergast R. A. Reactive lymphocytes in lacrimal gland and vasculitic renal lesions of autoimmune MRL/lpr mice express L3T4. J Exp Med. 1987 Oct 1;166(4):1198–1203. doi: 10.1084/jem.166.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler H. S. A laboratory model for Sjögren's syndrome. Am J Pathol. 1968 Mar;52(3):671–685. [PMC free article] [PubMed] [Google Scholar]
- Keyes G. G., Vickers R. A., Kersey J. H. Immunopathology of Sjögren-like disease in NZB/HZW mice. J Oral Pathol. 1977 Sep;6(5):288–295. doi: 10.1111/j.1600-0714.1977.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Kojima A., Tanaka-Kojima Y., Sakakura T., Nishizuka Y. Spontaneous development of autoimmune thyroiditis in neonatally thymectomized mice. Lab Invest. 1976 Jun;34(6):550–557. [PubMed] [Google Scholar]
- Kurashima C., Hirokawa K. Age-related increase of focal lymphocytic infiltration in the human submandibular glands. J Oral Pathol. 1986 Mar;15(3):172–178. doi: 10.1111/j.1600-0714.1986.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Makinodan T., Kay M. M. Age influence on the immune system. Adv Immunol. 1980;29:287–330. doi: 10.1016/s0065-2776(08)60047-4. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Sakakura T. Ovarian dysgenesis induced by neonatal thymectomy in the mouse. Endocrinology. 1971 Sep;89(3):886–893. doi: 10.1210/endo-89-3-886. [DOI] [PubMed] [Google Scholar]
- Rowley M. J., Buchanan H., Mackay I. R. Reciprocal change with age in antibody to extrinsic and intrinsic antigens. Lancet. 1968 Jul 6;2(7558):24–26. doi: 10.1016/s0140-6736(68)92893-6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Fukuma K., Kuribayashi K., Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985 Jan 1;161(1):72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Takahashi T., Nishizuka Y. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J Exp Med. 1982 Dec 1;156(6):1565–1576. doi: 10.1084/jem.156.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre D., Segre M. Age-related changes in B and T lymphocytes and decline of humoral immune responsiveness in aged mice. Mech Ageing Dev. 1977 Mar-Apr;6(2):115–129. doi: 10.1016/0047-6374(77)90013-6. [DOI] [PubMed] [Google Scholar]
- Silverman D. A., Rose N. R. Spontaneous and methylcholanthrene-enhanced thyroiditis in BUF rats. I. The incidence and severity of the disease, and the genetics of susceptibility. J Immunol. 1975 Jan;114(1 Pt 1):145–147. [PubMed] [Google Scholar]
- Taguchi O., Nishizuka Y. Experimental autoimmune orchitis after neonatal thymectomy in the mouse. Clin Exp Immunol. 1981 Nov;46(2):425–434. [PMC free article] [PubMed] [Google Scholar]
- Taguchi O., Nishizuka Y., Sakakura T., Kojima A. Autoimmune oophoritis in thymectomized mice: detection of circulating antibodies against oocytes. Clin Exp Immunol. 1980 Jun;40(3):540–553. [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Ishikawa G. Experimental autoallergic sialadenitis in mice. Histopathological and ultrastructural studies. Virchows Arch A Pathol Anat Histopathol. 1983;400(2):143–154. doi: 10.1007/BF00585496. [DOI] [PubMed] [Google Scholar]
- Utsuyama M., Hirokawa K. Age-related changes of splenic T cells in mice--a flow cytometric analysis. Mech Ageing Dev. 1987 Sep 14;40(1):89–102. doi: 10.1016/0047-6374(87)90037-6. [DOI] [PubMed] [Google Scholar]
- Welch P., Rose N. R., Kite J. H., Jr Neonatal thymectomy increases spontaneous autoimmune thyroiditis. J Immunol. 1973 Feb;110(2):575–577. [PubMed] [Google Scholar]
- White S. C., Casarett G. W. Induction of experimental autoallergic sialadenitis. J Immunol. 1974 Jan;112(1):178–185. [PubMed] [Google Scholar]
- Yunis E. J., Greenberg L. J. Immunopathology of aging. Fed Proc. 1974 Sep;33(9):2017–2019. [PubMed] [Google Scholar]