Abstract
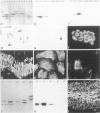
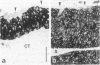
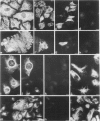
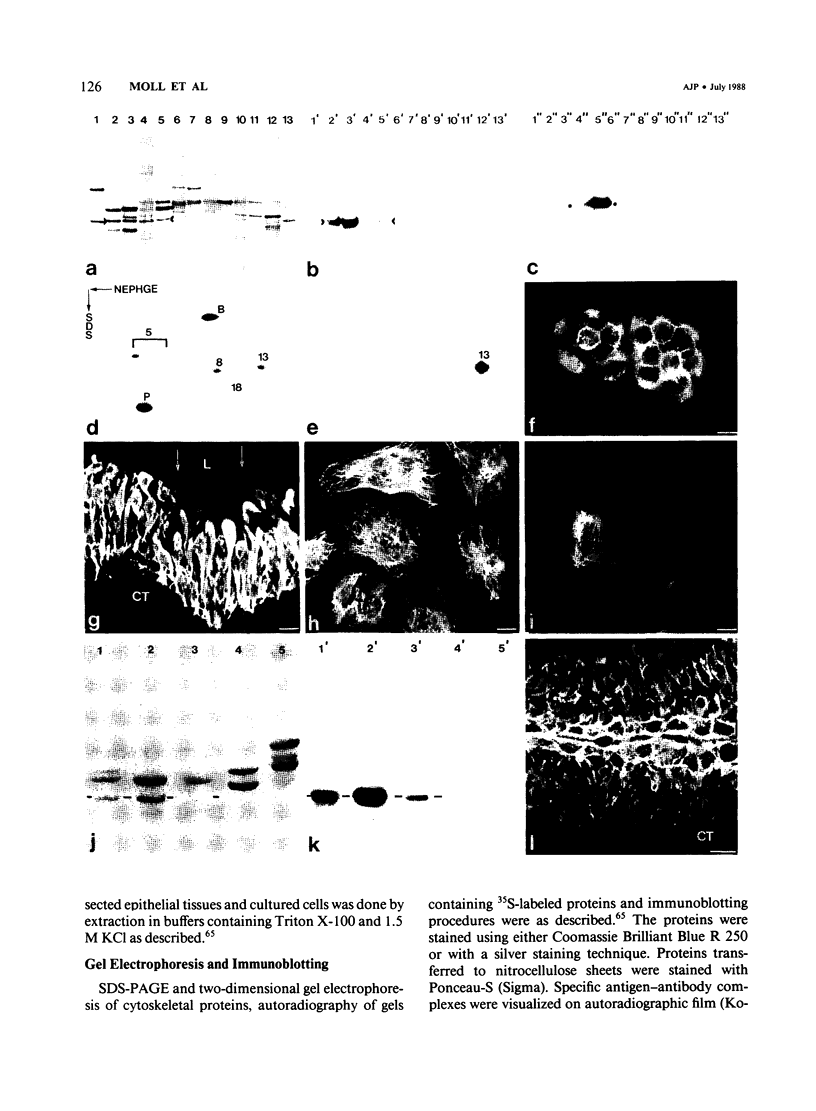
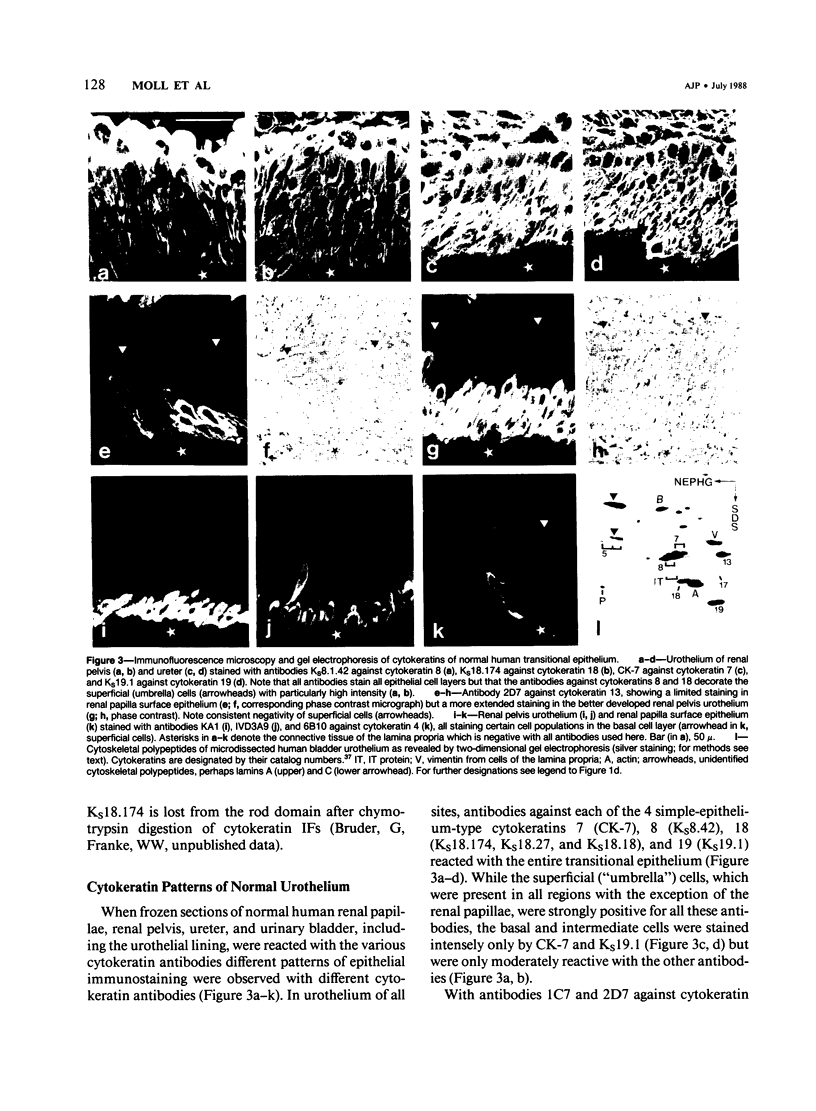
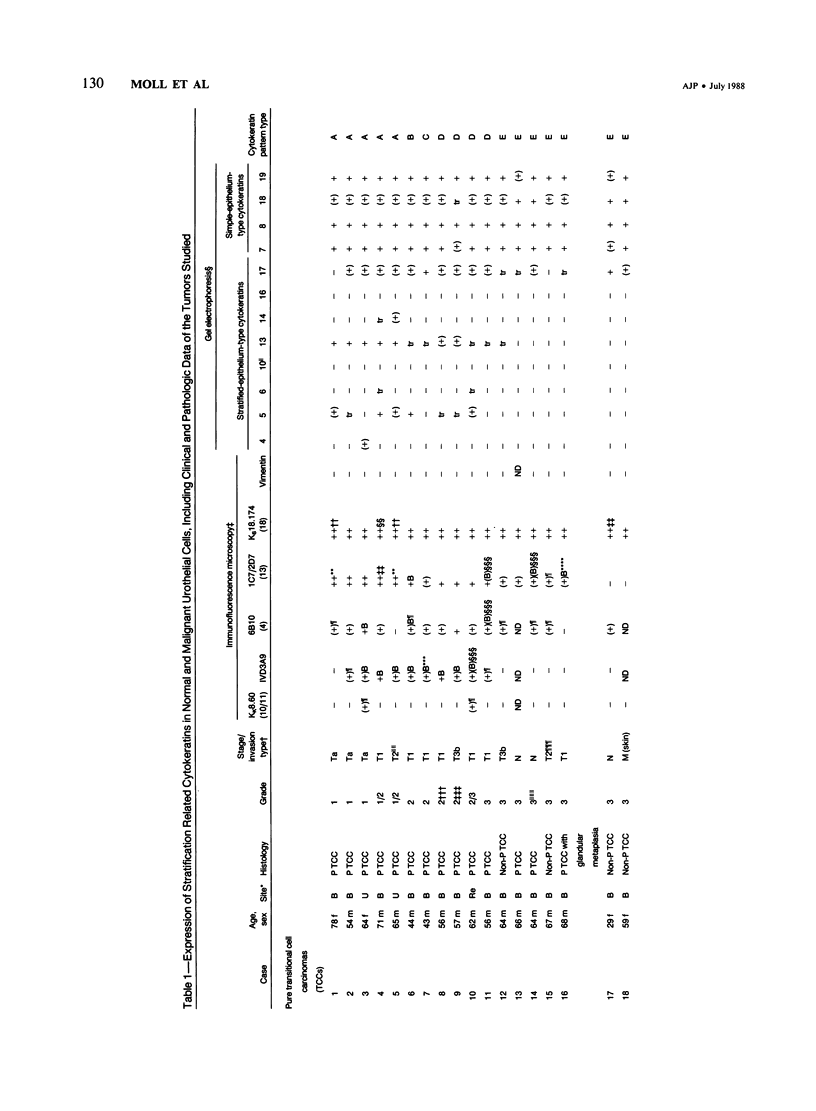
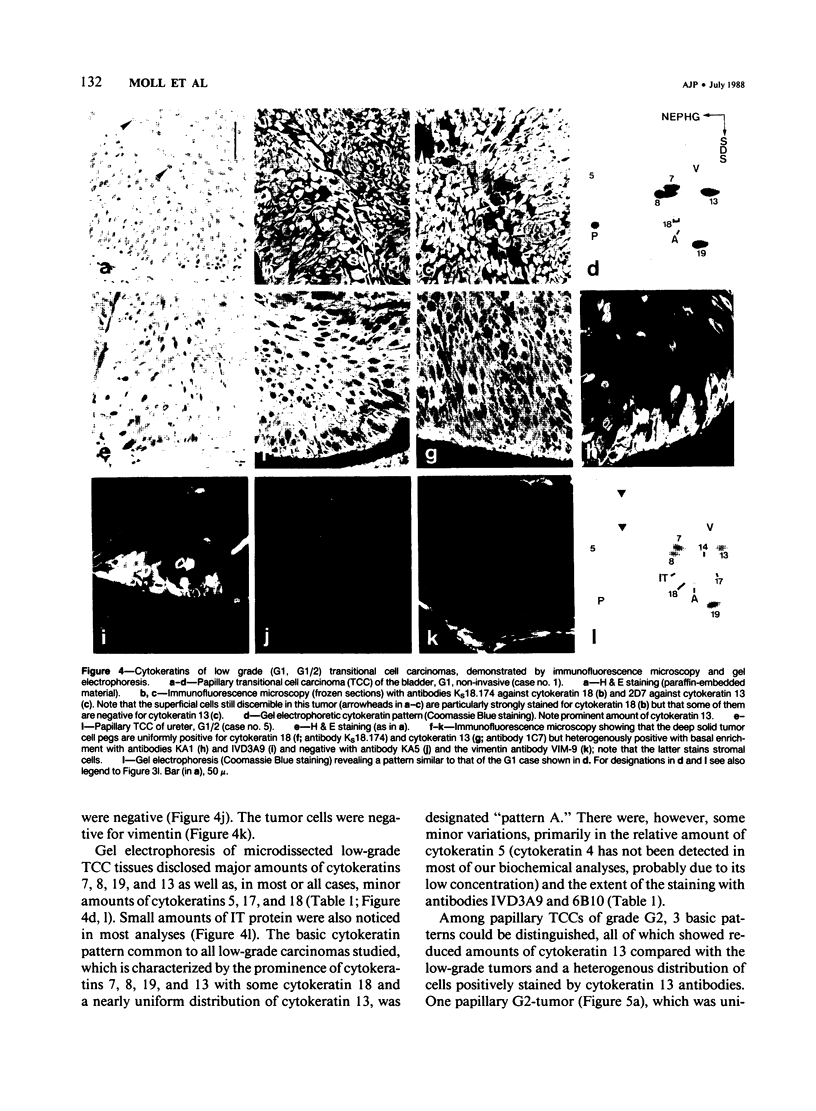
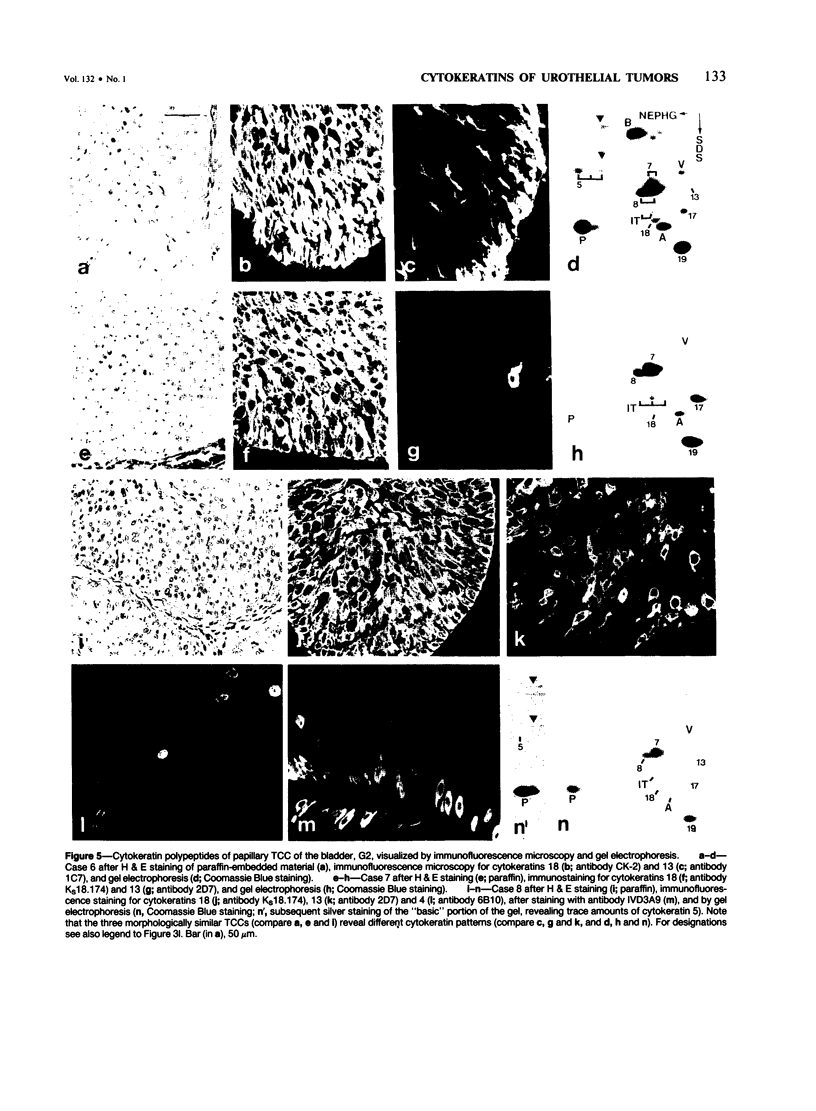
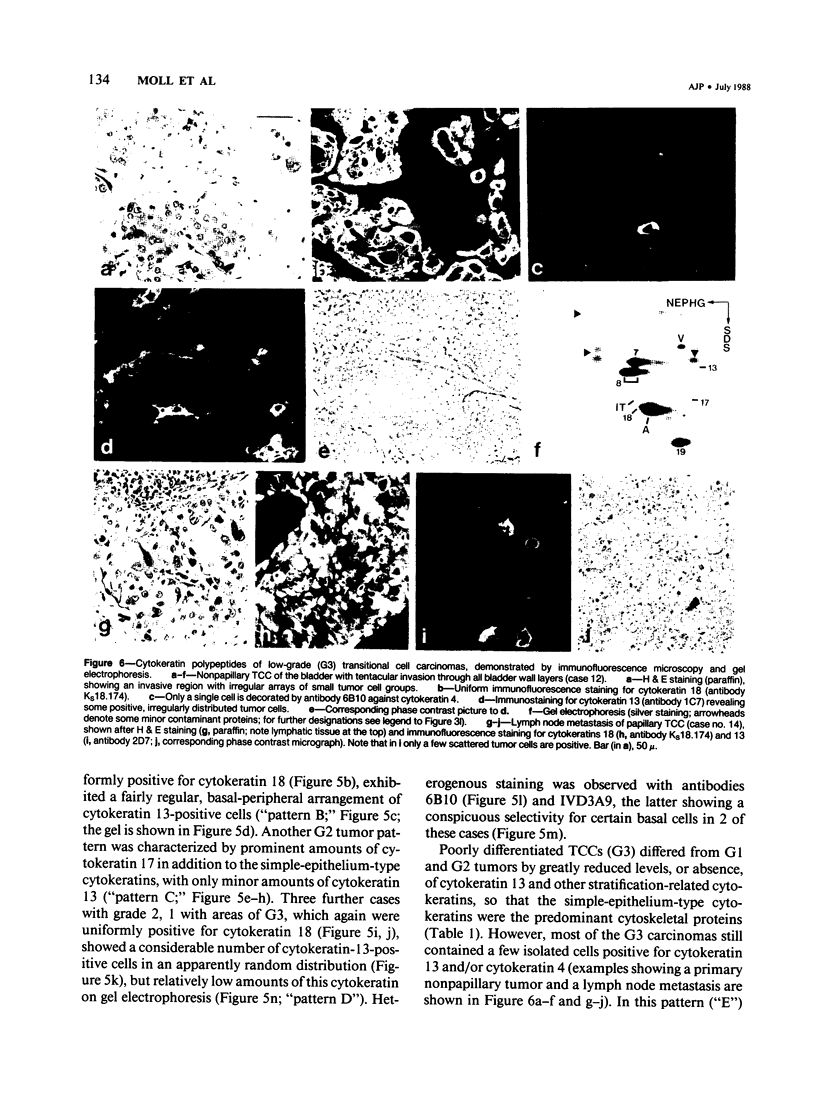
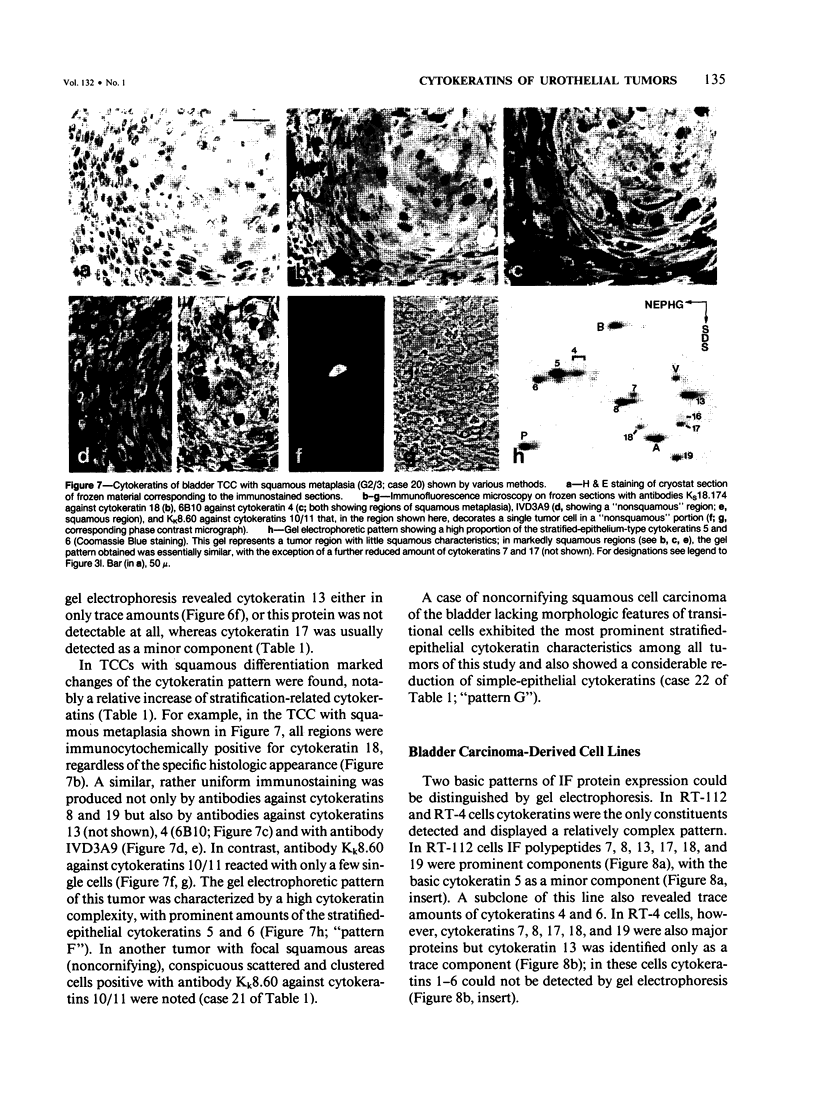
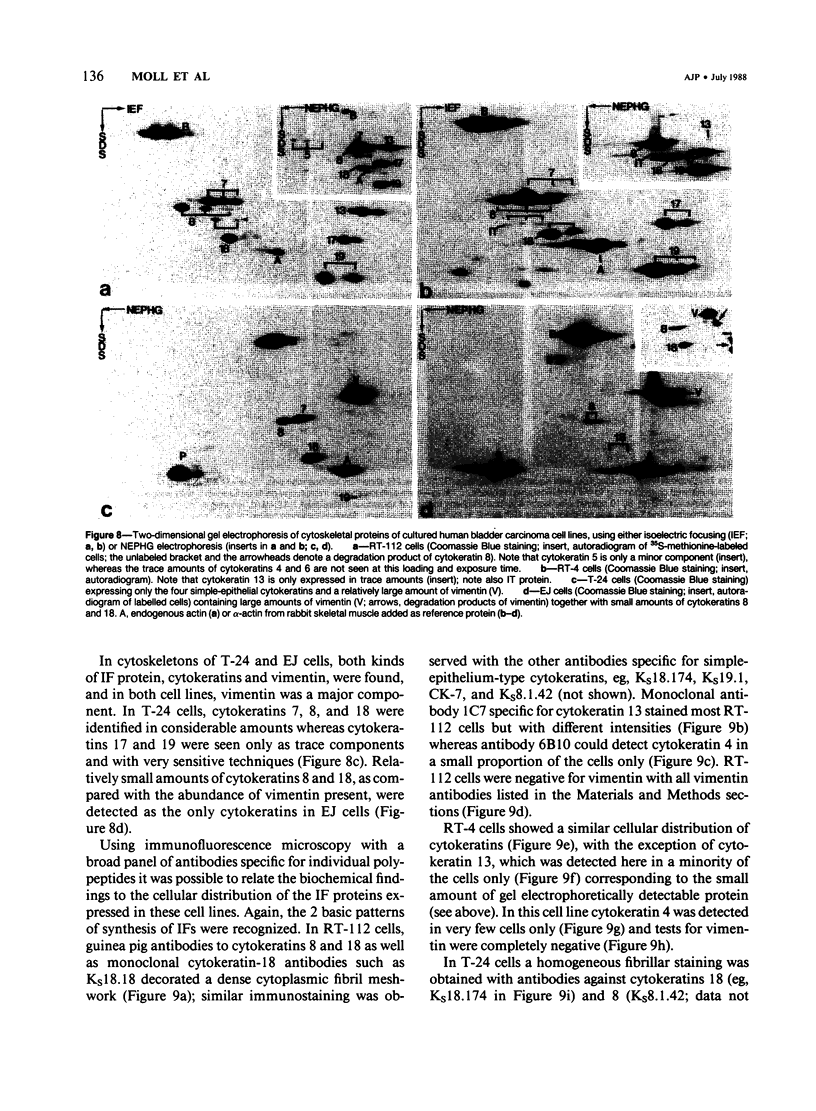
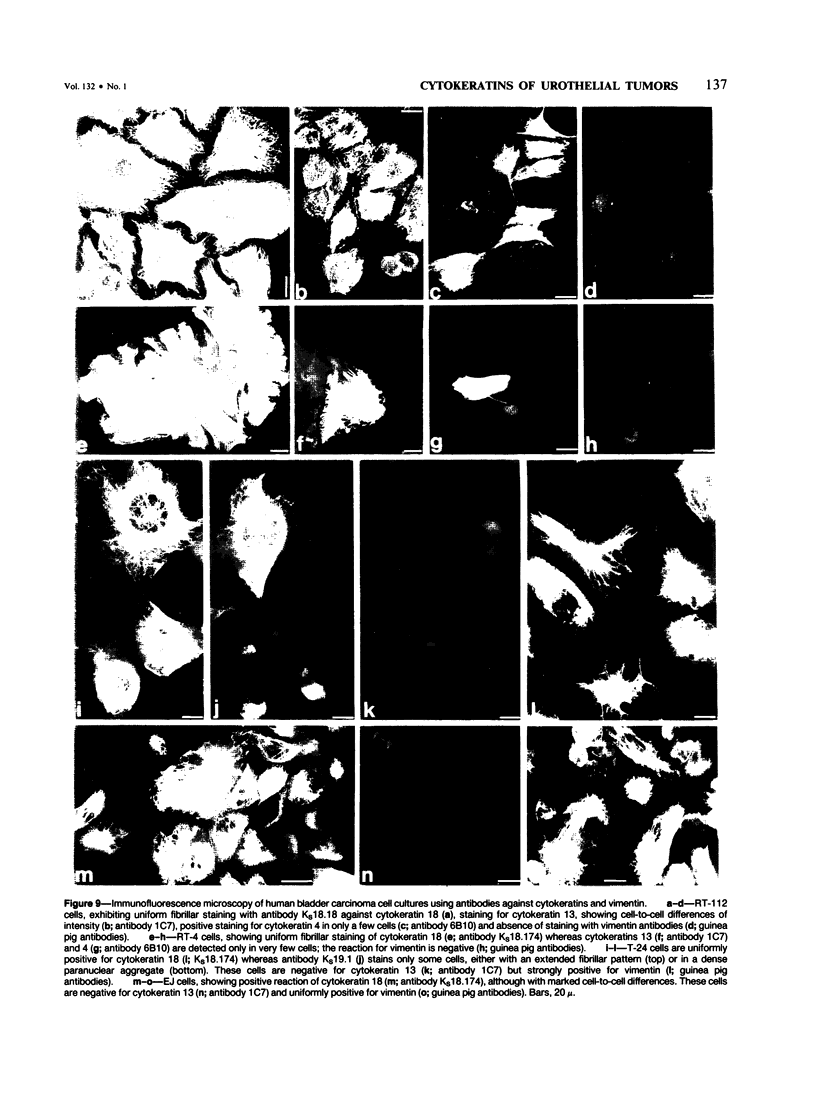
The pattern of cytokeratins expressed in normal urothelium has been compared with that of various forms of transitional cell carcinomas (TCCs; 21 cases) and cultured bladder carcinoma cell lines, using immunolocalization and gel electrophoretic techniques. In normal urothelium, all simple-epithelium-type cytokeratins (polypeptides 7, 8, 18, 19) were detected in all cell layers, whereas antibodies to cytokeratins typical for stratified epithelia reacted with certain basal cells only or, in the case of cytokeratin 13, with cells of the basal and intermediate layers. This pattern was essentially maintained in low-grade (G1, G1/2) TCCs but was remarkably modified in G2 TCCs. In G3 TCCs simple-epithelial cytokeratins were predominant whereas the amounts of component 13 were greatly reduced. Squamous metaplasia was accompanied generally by increased or new expression of some stratified-epithelial cytokeratins. The cytokeratin patterns of cell culture lines RT-112 and RT-4 resembled those of G1 and G2 TCCs, whereas cell line T-24 was comparable to G3 carcinomas. The cell line EJ showed a markedly different pattern. The results indicate that, in the cell layers of the urothelium, the synthesis of stratification-related cytokeratins such as component 13 is inversely oriented compared with that in other stratified epithelia where these proteins are suprabasally expressed, that TCCs retain certain intrinsic cytoskeletal features of urothelium, and that different TCCs can be distinguished by their cytokeratin patterns. The potential value of these observations in histopathologic and cytologic diagnoses is discussed.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstätter T., Moll R., Anderson A., Kuhn C., Pitz S., Schwechheimer K., Franke W. W. Expression of glial filament protein (GFP) in nerve sheaths and non-neural cells re-examined using monoclonal antibodies, with special emphasis on the co-expression of GFP and cytokeratins in epithelial cells of human salivary gland and pleomorphic adenomas. Differentiation. 1986;31(3):206–227. doi: 10.1111/j.1432-0436.1986.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Achtstaetter T., Hatzfeld M., Quinlan R. A., Parmelee D. C., Franke W. W. Separation of cytokeratin polypeptides by gel electrophoretic and chromatographic techniques and their identification by immunoblotting. Methods Enzymol. 1986;134:355–371. doi: 10.1016/0076-6879(86)34102-8. [DOI] [PubMed] [Google Scholar]
- Achtstätter T., Moll R., Moore B., Franke W. W. Cytokeratin polypeptide patterns of different epithelia of the human male urogenital tract: immunofluorescence and gel electrophoretic studies. J Histochem Cytochem. 1985 May;33(5):415–426. doi: 10.1177/33.5.2580881. [DOI] [PubMed] [Google Scholar]
- Battifora H., Sun T. T., Bahu R. M., Rao S. The use of antikeratin antiserum as a diagnostic tool: thymoma versus lymphoma. Hum Pathol. 1980 Nov;11(6):635–641. doi: 10.1016/s0046-8177(80)80074-8. [DOI] [PubMed] [Google Scholar]
- Blobel G. A., Moll R., Franke W. W., Vogt-Moykopf I. Cytokeratins in normal lung and lung carcinomas. I. Adenocarcinomas, squamous cell carcinomas and cultured cell lines. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(4):407–429. doi: 10.1007/BF02889883. [DOI] [PubMed] [Google Scholar]
- Bubeník J., Baresová M., Viklický V., Jakoubková J., Sainerová H., Donner J. Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int J Cancer. 1973 May;11(3):765–773. doi: 10.1002/ijc.2910110327. [DOI] [PubMed] [Google Scholar]
- Bártek J., Bártková J., Taylor-Papadimitriou J., Rejthar A., Kovarík J., Lukás Z., Vojtesek B. Differential expression of keratin 19 in normal human epithelial tissues revealed by monospecific monoclonal antibodies. Histochem J. 1986 Oct;18(10):565–575. doi: 10.1007/BF01675198. [DOI] [PubMed] [Google Scholar]
- Cintorino M., Del Vecchio M. T., Bugnoli M., Petracca R., Leoncini P. Cytokeratin pattern in normal and pathological bladder urothelium: immunohistochemical investigation using monoclonal antibodies. J Urol. 1988 Feb;139(2):428–432. doi: 10.1016/s0022-5347(17)42449-9. [DOI] [PubMed] [Google Scholar]
- Cooper D., Schermer A., Sun T. T. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985 Mar;52(3):243–256. [PubMed] [Google Scholar]
- Cordon-Cardo C., Finstad C. L., Bander N. H., Melamed M. R. Immunoanatomic distribution of cytostructural and tissue-associated antigens in the human urinary tract. Am J Pathol. 1987 Feb;126(2):269–284. [PMC free article] [PubMed] [Google Scholar]
- Cowin P., Kapprell H. P., Franke W. W. The complement of desmosomal plaque proteins in different cell types. J Cell Biol. 1985 Oct;101(4):1442–1454. doi: 10.1083/jcb.101.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. H., Ludwig M. E., Cole S. R., Pastuszak W. T. Small cell neuroendocrine carcinoma of the urinary bladder: report of three cases with ultrastructural analysis. Ultrastruct Pathol. 1983 Mar-Apr;4(2-3):197–204. doi: 10.3109/01913128309140790. [DOI] [PubMed] [Google Scholar]
- Debus E., Moll R., Franke W. W., Weber K., Osborn M. Immunohistochemical distinction of human carcinomas by cytokeratin typing with monoclonal antibodies. Am J Pathol. 1984 Jan;114(1):121–130. [PMC free article] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25(2):193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal antibodies to desmin, the muscle-specific intermediate filament protein. EMBO J. 1983;2(12):2305–2312. doi: 10.1002/j.1460-2075.1983.tb01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal cytokeratin antibodies that distinguish simple from stratified squamous epithelia: characterization on human tissues. EMBO J. 1982;1(12):1641–1647. doi: 10.1002/j.1460-2075.1982.tb01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feitz W. F., Beck H. L., Smeets A. W., Debruyne F. M., Vooijs G. P., Herman C. J., Ramaekers F. C. Tissue-specific markers in flow cytometry of urological cancers: cytokeratins in bladder carcinoma. Int J Cancer. 1985 Sep 15;36(3):349–356. [PubMed] [Google Scholar]
- Felix J. S., Littlefield J. W. Urinary tract epithelial cells cultured from human urine. Int Rev Cytol Suppl. 1979;(10):11–23. doi: 10.1016/s0074-7696(08)60609-9. [DOI] [PubMed] [Google Scholar]
- Felix J. S., Sun T. T., Littlefield J. W. Human epithelial cells cultured from urine: growth properties and keratin staining. In Vitro. 1980 Oct;16(10):866–874. doi: 10.1007/BF02619424. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Appelhans B., Schmid E., Freudenstein C., Osborn M., Weber K. Identification and characterization of epithelial cells in mammalian tissues by immunofluorescence microscopy using antibodies to prekeratin. Differentiation. 1979;15(1):7–25. doi: 10.1111/j.1432-0436.1979.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Mayer D., Schmid E., Denk H., Borenfreund E. Differences of expression of cytoskeletal proteins in cultured rat hepatocytes and hepatoma cells. Exp Cell Res. 1981 Aug;134(2):345–365. doi: 10.1016/0014-4827(81)90435-3. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Weber K., Osborn M. HeLa cells contain intermediate-sized filaments of the prekeratin type. Exp Cell Res. 1979 Jan;118(1):95–109. doi: 10.1016/0014-4827(79)90587-1. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Winter S., Schmid E., Söllner P., Hämmerling G., Achtstätter T. Monoclonal cytokeratin antibody recognizing a heterotypic complex: immunological probing of conformational states of cytoskeletal proteins in filaments and in solution. Exp Cell Res. 1987 Nov;173(1):17–37. doi: 10.1016/0014-4827(87)90328-4. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Fukushima S., Ito N., el-Bolkainy M. N., Tawfik H. N., Tatemoto Y., Mori M. Immunohistochemical observations of keratins, involucrin, and epithelial membrane antigen in urinary bladder carcinomas from patients infected with Schistosoma haematobium. Virchows Arch A Pathol Anat Histopathol. 1987;411(2):103–115. doi: 10.1007/BF00712734. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Kapanci Y., Barazzone P., Franke W. W. Immunochemical identification of intermediate-sized filaments in human neoplastic cells. A diagnostic aid for the surgical pathologist. Am J Pathol. 1981 Sep;104(3):206–216. [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M., Shimizu K., Perucho M., Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982 Apr 1;296(5856):404–409. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. III. Analysis of tumors. Am J Clin Pathol. 1985 Oct;84(4):413–424. doi: 10.1093/ajcp/84.4.413. [DOI] [PubMed] [Google Scholar]
- Hasegawa R., Cohen S. M. Immunohistochemical study of keratin in proliferative bladder epithelium induced by freezing, cyclophosphamide or N-[4-(5-nitro-2-furyl)-2-thiazolyl]formamide in the rat. Carcinogenesis. 1985 Mar;6(3):409–414. doi: 10.1093/carcin/6.3.409. [DOI] [PubMed] [Google Scholar]
- Hastings R. J., Franks L. M. Cellular heterogeneity in a tissue culture cell line derived from a human bladder carcinoma. Br J Cancer. 1983 Feb;47(2):233–244. doi: 10.1038/bjc.1983.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld M., Franke W. W. Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate-sized filaments by homologous and heterologous recombinations of purified polypeptides. J Cell Biol. 1985 Nov;101(5 Pt 1):1826–1841. doi: 10.1083/jcb.101.5.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman C. J., Vegt P. D., Debruyne F. M., Vooijs G. P., Ramaekers F. C. Squamous and transitional elements in rat bladder carcinomas induced by N-butyl-N-4-hydroxybutyl-nitrosamine (BBN). A study of cytokeratin expression. Am J Pathol. 1985 Sep;120(3):419–426. [PMC free article] [PubMed] [Google Scholar]
- Herz F., Schermer A., Koss L. G. Short-term culture of epithelial cells from urine of adults. Proc Soc Exp Biol Med. 1979 Jun;161(2):153–157. doi: 10.3181/00379727-161-40509. [DOI] [PubMed] [Google Scholar]
- Hicks R. M., Chowaniec J. Experimental induction, histology, and ultrastructure of hyperplasia and neoplasia of the urinary bladder epithelium. Int Rev Exp Pathol. 1978;18:199–280. [PubMed] [Google Scholar]
- Hicks R. M. The mammalian urinary bladder: an accommodating organ. Biol Rev Camb Philos Soc. 1975 May;50(2):215–246. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Hicks R. M. The permeability of rat transitional epithelium. Kertinization and the barrier to water. J Cell Biol. 1966 Jan;28(1):21–31. doi: 10.1083/jcb.28.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar M., Gigi-Leitner O., Moll R., Franke W. W., Geiger B. Monoclonal antibodies to various acidic (type I) cytokeratins of stratified epithelia. Selective markers for stratification and squamous cell carcinomas. Differentiation. 1986;31(2):141–153. doi: 10.1111/j.1432-0436.1986.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Kern W. H. The grade and pathologic stage of bladder cancer. Cancer. 1984 Mar 1;53(5):1185–1189. doi: 10.1002/1097-0142(19840301)53:5<1185::aid-cncr2820530526>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Kieler J. F. Invasiveness of transformed bladder epithelial cells. Cancer Metastasis Rev. 1984;3(3):265–296. doi: 10.1007/BF00048389. [DOI] [PubMed] [Google Scholar]
- Kopan R., Traska G., Fuchs E. Retinoids as important regulators of terminal differentiation: examining keratin expression in individual epidermal cells at various stages of keratinization. J Cell Biol. 1987 Jul;105(1):427–440. doi: 10.1083/jcb.105.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss L. G. Some ultrastructural aspects of experimental and human carcinoma of the bladder. Cancer Res. 1977 Aug;37(8 Pt 2):2824–2835. [PubMed] [Google Scholar]
- Koss L. G. The asymmetric unit membranes of the epithelium of the urinary bladder of the rat. An electron microscopic study of a mechanism of epithelial maturation and function. Lab Invest. 1969 Aug;21(2):154–168. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Leube R. E., Bosch F. X., Romano V., Zimbelmann R., Höfler H., Franke W. W. Cytokeratin expression in simple epithelia. III. Detection of mRNAs encoding human cytokeratins nos. 8 and 18 in normal and tumor cells by hybridization with cDNA sequences in vitro and in situ. Differentiation. 1986;33(1):69–85. doi: 10.1111/j.1432-0436.1986.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Linder D. Culture of cells from the urine and bladder washings of adults. Somatic Cell Genet. 1976 May;2(3):281–283. doi: 10.1007/BF01538966. [DOI] [PubMed] [Google Scholar]
- Mills S. E., Wolfe J. T., 3rd, Weiss M. A., Swanson P. E., Wick M. R., Fowler J. E., Jr, Young R. H. Small cell undifferentiated carcinoma of the urinary bladder. A light-microscopic, immunocytochemical, and ultrastructural study of 12 cases. Am J Surg Pathol. 1987 Aug;11(8):606–617. doi: 10.1097/00000478-198708000-00004. [DOI] [PubMed] [Google Scholar]
- Moll R., Cowin P., Kapprell H. P., Franke W. W. Desmosomal proteins: new markers for identification and classification of tumors. Lab Invest. 1986 Jan;54(1):4–25. [PubMed] [Google Scholar]
- Moll R., Franke W. W. Cytoskeletal differences between human neuroendocrine tumors: a cytoskeletal protein of molecular weight 46,000 distinguishes cutaneous from pulmonary neuroendocrine neoplasms. Differentiation. 1985;30(2):165–175. doi: 10.1111/j.1432-0436.1985.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Krepler R., Franke W. W. Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation. 1983;23(3):256–269. doi: 10.1111/j.1432-0436.1982.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Moll R., Levy R., Czernobilsky B., Hohlweg-Majert P., Dallenbach-Hellweg G., Franke W. W. Cytokeratins of normal epithelia and some neoplasms of the female genital tract. Lab Invest. 1983 Nov;49(5):599–610. [PubMed] [Google Scholar]
- Nadakavukaren K. K., Summerhayes I. C., Salcedo B. F., Rheinwald J. G., Chen L. B. A monoclonal antibody recognizing a keratin filament protein in a subset of transitional and glandular epithelia. Differentiation. 1984;27(3):209–220. doi: 10.1111/j.1432-0436.1984.tb01431.x. [DOI] [PubMed] [Google Scholar]
- Nagle R. B., Böcker W., Davis J. R., Heid H. W., Kaufmann M., Lucas D. O., Jarasch E. D. Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J Histochem Cytochem. 1986 Jul;34(7):869–881. doi: 10.1177/34.7.2423579. [DOI] [PubMed] [Google Scholar]
- Nathrath W. B., Heidenkummer P., Björklund V., Björklund B. Distribution of tissue polypeptide antigen (TPA) in normal human tissues: Immunohistochemical study on unfixed, methanol-, ethanol-, and formalin-fixed tissues. J Histochem Cytochem. 1985 Feb;33(2):99–109. doi: 10.1177/33.2.3968422. [DOI] [PubMed] [Google Scholar]
- O'Toole C. M., Povey S., Hepburn P., Franks L. M. Identity of some human bladder cancer cell lines. Nature. 1983 Feb 3;301(5899):429–430. doi: 10.1038/301429a0. [DOI] [PubMed] [Google Scholar]
- Osborn M., Debus E., Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol. 1984 May;34(1):137–143. [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Petry G., Amon H. Licht- und elektronenmikroskopische Studien über Struktur und Dynamik des Ubergangsepithels. Z Zellforsch Mikrosk Anat. 1966;69:587–612. [PubMed] [Google Scholar]
- Povey S., Hopkinson D. A., Harris H., Franks L. M. Characterisation of human cell lines and differentiation from HeLa by enzyme typing. Nature. 1976 Nov 4;264(5581):60–63. doi: 10.1038/264060b0. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Moesker O., Schaart G., Herman C., Vooijs P. Cytokeratin expression during neoplastic progression of human transitional cell carcinomas as detected by a monoclonal and a polyclonal antibody. Lab Invest. 1985 Jan;52(1):31–38. [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Schaart G., Moesker O., Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987 May;170(1):235–249. doi: 10.1016/0014-4827(87)90133-9. [DOI] [PubMed] [Google Scholar]
- Rigby C. C., Franks L. M. A human tissue culture cell line from a transitional cell tumour of the urinary bladder: growth, chromosone pattern and ultrastructure. Br J Cancer. 1970 Dec;24(4):746–754. doi: 10.1038/bjc.1970.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid E., Schiller D. L., Grund C., Stadler J., Franke W. W. Tissue type-specific expression of intermediate filament proteins in a cultured epithelial cell line from bovine mammary gland. J Cell Biol. 1983 Jan;96(1):37–50. doi: 10.1083/jcb.96.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatore S., Attolini A., Luccarelli S., Candita F., Trabucco M. Tissue polypeptide antigen in normal and neoplastic urinary bladder. Preliminary reports. Oncology. 1987;44(2):118–123. doi: 10.1159/000226458. [DOI] [PubMed] [Google Scholar]
- Settle S. A., Hellström I., Hellström K. E. A monoclonal antibody recognizing cytoskeletal keratins of stratified epithelia and bladder carcinomas. Exp Cell Res. 1985 Apr;157(2):293–306. doi: 10.1016/0014-4827(85)90114-4. [DOI] [PubMed] [Google Scholar]
- Summerhayes I. C., Chen L. B. Localization of a Mr 52,000 keratin in basal epithelial cells of the mouse bladder and expression throughout neoplastic progression. Cancer Res. 1982 Oct;42(10):4098–4109. [PubMed] [Google Scholar]
- Summerhayes I. C., Cheng Y. S., Sun T. T., Chen L. B. Expression of keratin and vimentin intermediate filaments in rabbit bladder epithelial cells at different stages of benzo[a]pyrene-induced neoplastic progression. J Cell Biol. 1981 Jul;90(1):63–69. doi: 10.1083/jcb.90.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Shih C., Green H. Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2813–2817. doi: 10.1073/pnas.76.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Tseng S. C., Huang A. J., Cooper D., Schermer A., Lynch M. H., Weiss R., Eichner R. Monoclonal antibody studies of mammalian epithelial keratins: a review. Ann N Y Acad Sci. 1985;455:307–329. doi: 10.1111/j.1749-6632.1985.tb50419.x. [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Jarvinen M. J., Nelson W. G., Huang J. W., Woodcock-Mitchell J., Sun T. T. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982 Sep;30(2):361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- Tölle H. G., Weber K., Osborn M. Microinjection of monoclonal antibodies specific for one intermediate filament protein in cells containing multiple keratins allow insight into the composition of particular 10 nm filaments. Eur J Cell Biol. 1985 Sep;38(2):234–244. [PubMed] [Google Scholar]
- Venetianer A., Schiller D. L., Magin T., Franke W. W. Cessation of cytokeratin expression in a rat hepatoma cell line lacking differentiated functions. Nature. 1983 Oct 20;305(5936):730–733. doi: 10.1038/305730a0. [DOI] [PubMed] [Google Scholar]
- Wu Y. J., Parker L. M., Binder N. E., Beckett M. A., Sinard J. H., Griffiths C. T., Rheinwald J. G. The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell. 1982 Dec;31(3 Pt 2):693–703. doi: 10.1016/0092-8674(82)90324-5. [DOI] [PubMed] [Google Scholar]
- van Muijen G. N., Ruiter D. J., Franke W. W., Achtstätter T., Haasnoot W. H., Ponec M., Warnaar S. O. Cell type heterogeneity of cytokeratin expression in complex epithelia and carcinomas as demonstrated by monoclonal antibodies specific for cytokeratins nos. 4 and 13. Exp Cell Res. 1986 Jan;162(1):97–113. doi: 10.1016/0014-4827(86)90429-5. [DOI] [PubMed] [Google Scholar]