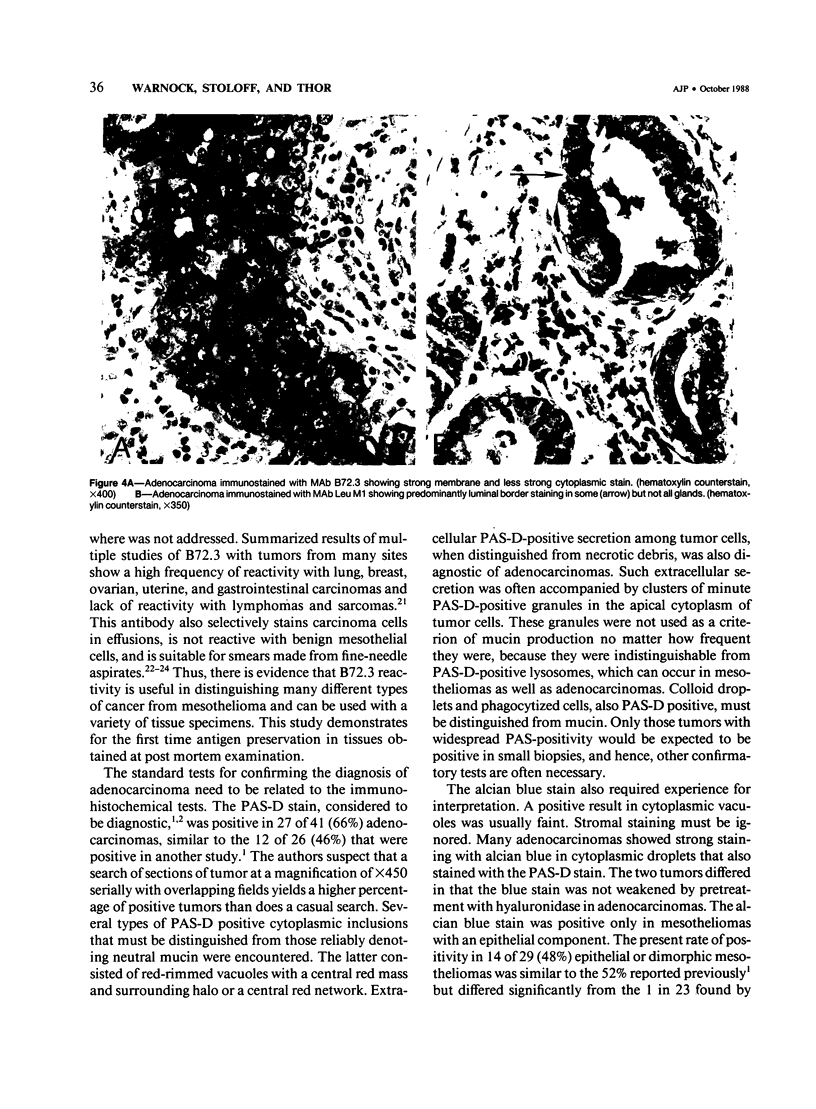
Abstract
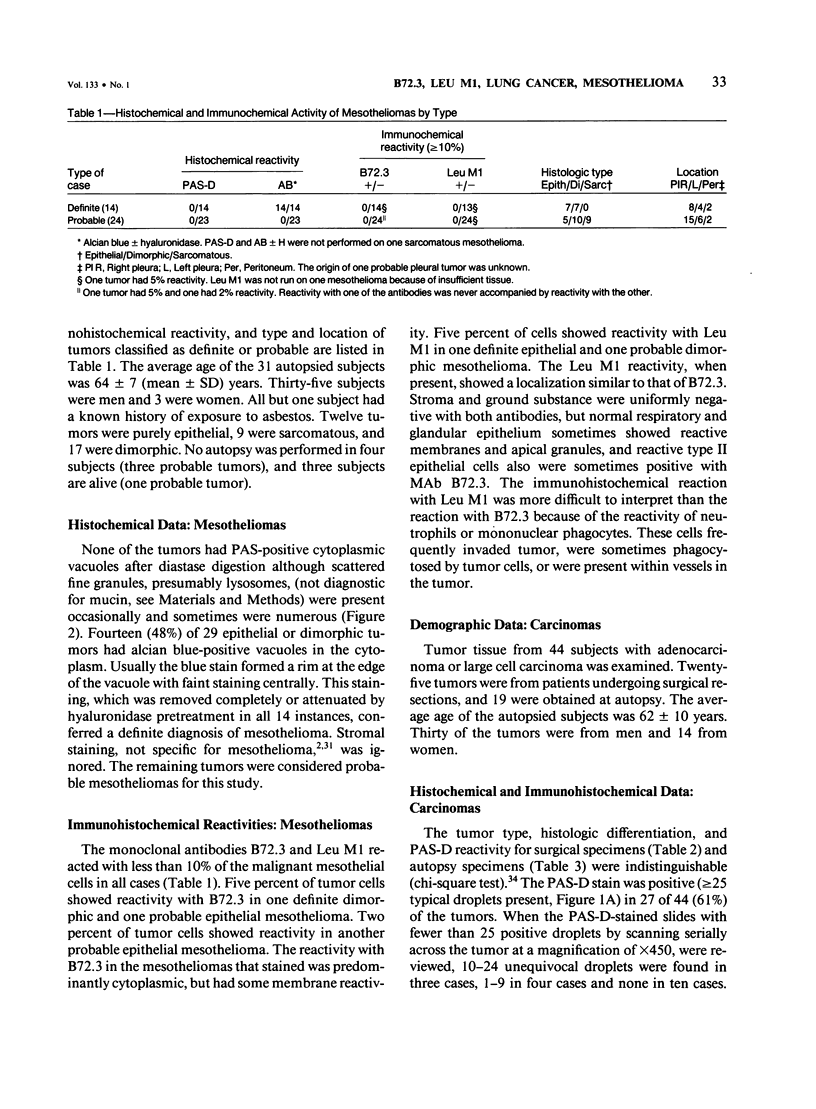
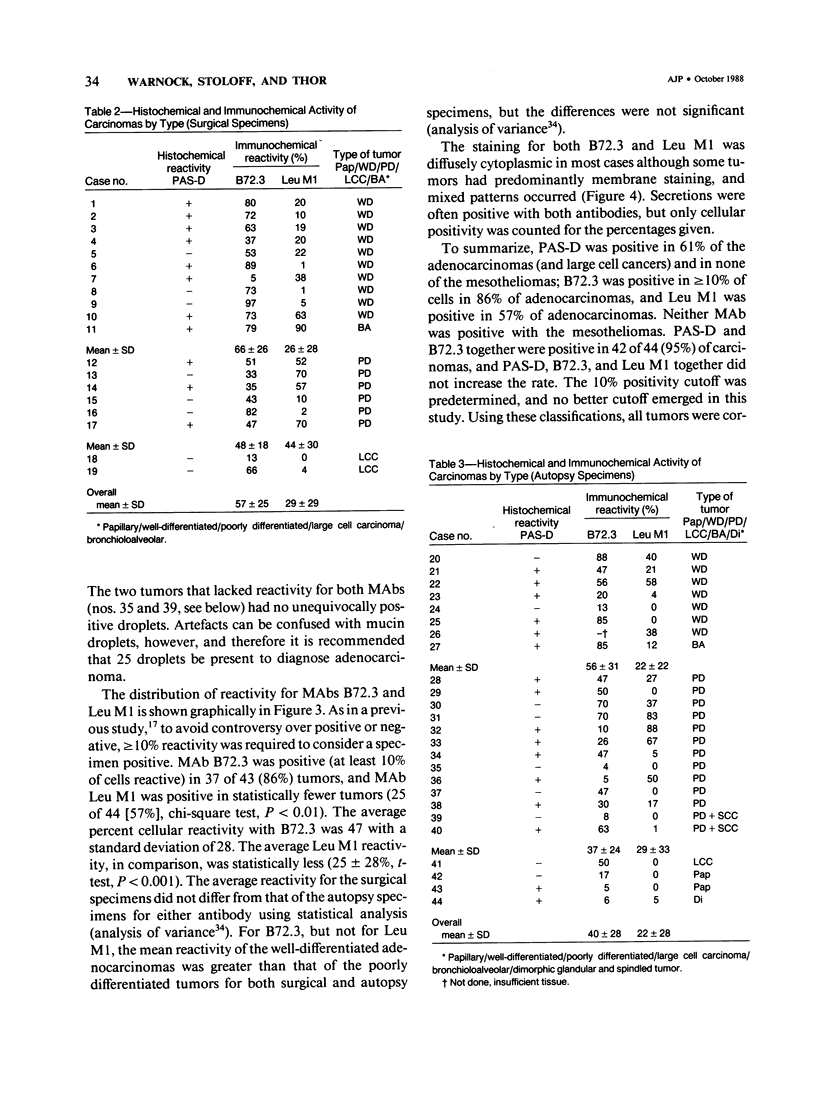
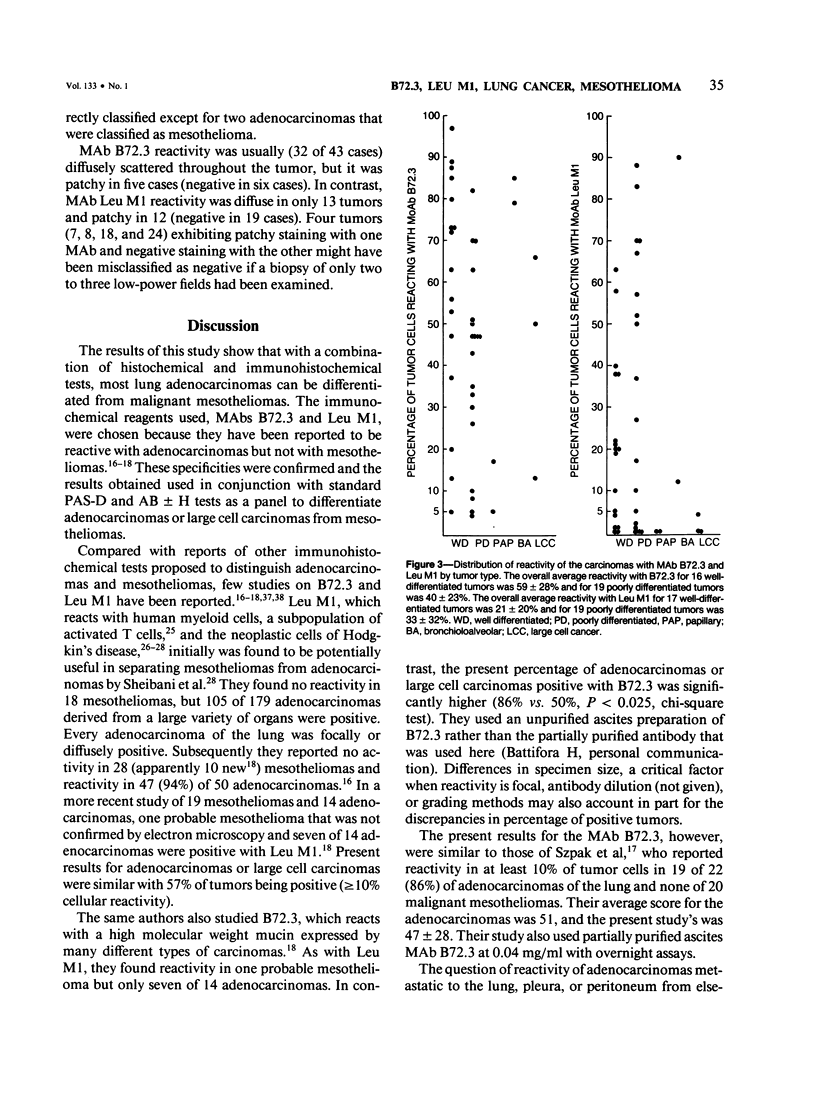
The immunohistochemical reactivity of 38 mesotheliomas and 44 adeno-carcinomas or large cell carcinomas of the lung with monoclonal antibodies (MAb) B72.3 and Leu M1 was compared with their reactivity with the routine histochemic stains periodic acid-Schiff with diastase digestion (PAS-D) and alcian blue +/- hyaluronidase. Both MAbs reacted selectively with carcinomas when a positive test was set at greater than or equal to 10% reactive tumor cells. However, MAb B72.3 reacted with significantly more of the carcinomas (86%, chi-square test, P less than 0.01) and bound to a greater percentage of tumor cells (47 +/- 28%; mean +/- SD, t-test, P less than 0.001) than Leu M1 (57% and 25 +/- 28%, respectively). The similar reactivities of surgically resected tumor specimens and post mortem tissues with both antibodies confirmed antigen stability and suggested broad clinical utility. PAS-D stained 61% of the carcinomas. Using the markers for carcinomas (PAS-D, B72.3, and Leu M1), the tumors were classified into the correct group in 80 of 82 (98%) cases (95% confidence level: greater than 92% accuracy). The alcian blue stain was useful to confirm a diagnosis of dimorphic or epithelial mesothelioma (48% were positive).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams V. I., Unni K. K. Diffuse malignant mesothelioma of pleura: diagnostic criteria based on an autopsy study. Am J Clin Pathol. 1984 Jul;82(1):15–23. doi: 10.1093/ajcp/82.1.15. [DOI] [PubMed] [Google Scholar]
- Battifora H., Kopinski M. I. Distinction of mesothelioma from adenocarcinoma. An immunohistochemical approach. Cancer. 1985 Apr 15;55(8):1679–1685. doi: 10.1002/1097-0142(19850415)55:8<1679::aid-cncr2820550812>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bolen J. W., Thorning D. Mesotheliomas: a light- and electron-microscopical study concerning histogenetic relationships between the epithelial and the mesenchymal variants. Am J Surg Pathol. 1980 Oct;4(5):451–464. [PubMed] [Google Scholar]
- Churg A. Immunohistochemical staining for vimentin and keratin in malignant mesothelioma. Am J Surg Pathol. 1985 May;9(5):360–365. doi: 10.1097/00000478-198505000-00006. [DOI] [PubMed] [Google Scholar]
- Cibas E. S., Corson J. M., Pinkus G. S. The distinction of adenocarcinoma from malignant mesothelioma in cell blocks of effusions: the role of routine mucin histochemistry and immunohistochemical assessment of carcinoembryonic antigen, keratin proteins, epithelial membrane antigen, and milk fat globule-derived antigen. Hum Pathol. 1987 Jan;18(1):67–74. doi: 10.1016/s0046-8177(87)80196-x. [DOI] [PubMed] [Google Scholar]
- Colcher D., Hand P. H., Nuti M., Schlom J. A spectrum of monoclonal antibodies reactive with human mammary tumor cells. Proc Natl Acad Sci U S A. 1981 May;78(5):3199–3203. doi: 10.1073/pnas.78.5.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatter K. C., Dunnill M. S., Pulford K. A., Heryet A., Mason D. Y. Human lung tumours: a correlation of antigenic profile with histological type. Histopathology. 1985 Aug;9(8):805–823. doi: 10.1111/j.1365-2559.1985.tb02868.x. [DOI] [PubMed] [Google Scholar]
- Hanjan S. N., Kearney J. F., Cooper M. D. A monoclonal antibody (MMA) that identifies a differentiation antigen on human myelomonocytic cells. Clin Immunol Immunopathol. 1982 May;23(2):172–188. doi: 10.1016/0090-1229(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Holden J., Churg A. Immunohistochemical staining for keratin and carcinoembryonic antigen in the diagnosis of malignant mesothelioma. Am J Surg Pathol. 1984 Apr;8(4):277–279. doi: 10.1097/00000478-198404000-00004. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Leu M1 and peanut agglutinin stain the neoplastic cells of Hodgkin's disease. Am J Clin Pathol. 1984 Jul;82(1):29–32. doi: 10.1093/ajcp/82.1.29. [DOI] [PubMed] [Google Scholar]
- Johnson V. G., Schlom J., Paterson A. J., Bennett J., Magnani J. L., Colcher D. Analysis of a human tumor-associated glycoprotein (TAG-72) identified by monoclonal antibody B72.3. Cancer Res. 1986 Feb;46(2):850–857. [PubMed] [Google Scholar]
- Johnston W. W., Szpak C. A., Lottich S. C., Thor A., Schlom J. Use of a monoclonal antibody (B72.3) as a novel immunohistochemical adjunct for the diagnosis of carcinomas in fine needle aspiration biopsy specimens. Hum Pathol. 1986 May;17(5):501–513. doi: 10.1016/s0046-8177(86)80041-7. [DOI] [PubMed] [Google Scholar]
- Johnston W. W., Szpak C. A., Thor A., Schlom J. Phenotypic characterization of lung cancers in fine needle aspiration biopsies using monoclonal antibody B72.3. Cancer Res. 1986 Dec;46(12 Pt 1):6462–6470. [PubMed] [Google Scholar]
- Kannerstein M., Churg J., Magner D. Histochemistry in the diagnosis of malignant mesothelioma. Ann Clin Lab Sci. 1973 May-Jun;3(3):207–211. [PubMed] [Google Scholar]
- Kannerstein M., Churg J., McCaughey W. T. Asbestos and mesothelioma: a review. Pathol Annu. 1978;13(Pt 1):81–129. [PubMed] [Google Scholar]
- Kannerstein M., McCaughey W. T., Churg J., Selikoff I. J. A critique of the criteria for the diagnosis of diffuse malignant mesothelioma. Mt Sinai J Med. 1977 Jul-Aug;44(4):485–494. [PubMed] [Google Scholar]
- Kwee W. S., Veldhuizen R. W., Golding R. P., Mullink H., Stam J., Donner R., Boon M. E. Histologic distinction between malignant mesothelioma, benign pleural lesion and carcinoma metastasis. Evaluation of the application of morphometry combined with histochemistry and immunostaining. Virchows Arch A Pathol Anat Histol. 1982;397(3):287–299. doi: 10.1007/BF00496570. [DOI] [PubMed] [Google Scholar]
- Lauritzen A. F. Distinction between cells in serous effusions using a panel of antibodies. Virchows Arch A Pathol Anat Histopathol. 1987;411(3):299–304. doi: 10.1007/BF00735037. [DOI] [PubMed] [Google Scholar]
- Lee I., Radosevich J. A., Chejfec G., Ma Y. X., Warren W. H., Rosen S. T., Gould V. E. Malignant mesotheliomas. Improved differential diagnosis from lung adenocarcinomas using monoclonal antibodies 44-3A6 and 624A12. Am J Pathol. 1986 Jun;123(3):497–507. [PMC free article] [PubMed] [Google Scholar]
- Otis C. N., Carter D., Cole S., Battifora H. Immunohistochemical evaluation of pleural mesothelioma and pulmonary adenocarcinoma. A bi-institutional study of 47 cases. Am J Surg Pathol. 1987 Jun;11(6):445–456. doi: 10.1097/00000478-198706000-00005. [DOI] [PubMed] [Google Scholar]
- Pinkus G. S., Said J. W. Leu-M1 immunoreactivity in nonhematopoietic neoplasms and myeloproliferative disorders. An immunoperoxidase study of paraffin sections. Am J Clin Pathol. 1986 Mar;85(3):278–282. doi: 10.1093/ajcp/85.3.278. [DOI] [PubMed] [Google Scholar]
- Pinkus G. S., Thomas P., Said J. W. Leu-M1--a marker for Reed-Sternberg cells in Hodgkin's disease. An immunoperoxidase study of paraffin-embedded tissues. Am J Pathol. 1985 May;119(2):244–252. [PMC free article] [PubMed] [Google Scholar]
- Sewell H. F., Jaffray B., Thompson W. D. Reaction of monoclonal anti Leu M1--a myelomonocytic marker (CD15)--with normal and neoplastic epithelia. J Pathol. 1987 Apr;151(4):279–284. doi: 10.1002/path.1711510407. [DOI] [PubMed] [Google Scholar]
- Sheibani K., Battifora H., Burke J. S. Antigenic phenotype of malignant mesotheliomas and pulmonary adenocarcinomas. An immunohistologic analysis demonstrating the value of Leu M1 antigen. Am J Pathol. 1986 May;123(2):212–219. [PMC free article] [PubMed] [Google Scholar]
- Sheibani K., Battifora H., Burke J. S., Rappaport H. Leu-M1 antigen in human neoplasms. An immunohistologic study of 400 cases. Am J Surg Pathol. 1986 Apr;10(4):227–236. doi: 10.1097/00000478-198604000-00001. [DOI] [PubMed] [Google Scholar]
- Strickler J. G., Herndier B. G., Rouse R. V. Immunohistochemical staining in malignant mesotheliomas. Am J Clin Pathol. 1987 Nov;88(5):610–614. doi: 10.1093/ajcp/88.5.610. [DOI] [PubMed] [Google Scholar]
- Szpak C. A., Johnston W. W., Roggli V., Kolbeck J., Lottich S. C., Vollmer R., Thor A., Schlom J. The diagnostic distinction between malignant mesothelioma of the pleura and adenocarcinoma of the lung as defined by a monoclonal antibody (B72.3). Am J Pathol. 1986 Feb;122(2):252–260. [PMC free article] [PubMed] [Google Scholar]
- Tron V., Wright J. L., Churg A. Carcinoembryonic antigen and milk-fat globule protein staining of malignant mesothelioma and adenocarcinoma of the lung. Arch Pathol Lab Med. 1987 Mar;111(3):291–293. [PubMed] [Google Scholar]
- Warhol M. J., Corson J. M. An ultrastructural comparison of mesotheliomas with adenocarcinomas of the lung and breast. Hum Pathol. 1985 Jan;16(1):50–55. doi: 10.1016/s0046-8177(85)80213-6. [DOI] [PubMed] [Google Scholar]
- Warhol M. J., Hickey W. F., Corson J. M. Malignant mesothelioma: ultrastructural distinction from adenocarcinoma. Am J Surg Pathol. 1982 Jun;6(4):307–314. [PubMed] [Google Scholar]
- Warnock M. L., Wolery G. Asbestos bodies or fibers and the diagnosis of asbestosis. Environ Res. 1987 Oct;44(1):29–44. doi: 10.1016/s0013-9351(87)80084-1. [DOI] [PubMed] [Google Scholar]
- Yagasaki K., Jacobson S. G., Apáthy P. P., Knighton R. W. Rod and cone psychophysics and electroretinography: methods for comparison in retinal degenerations. Doc Ophthalmol. 1988 Jun;69(2):119–130. doi: 10.1007/BF00153692. [DOI] [PubMed] [Google Scholar]