Abstract
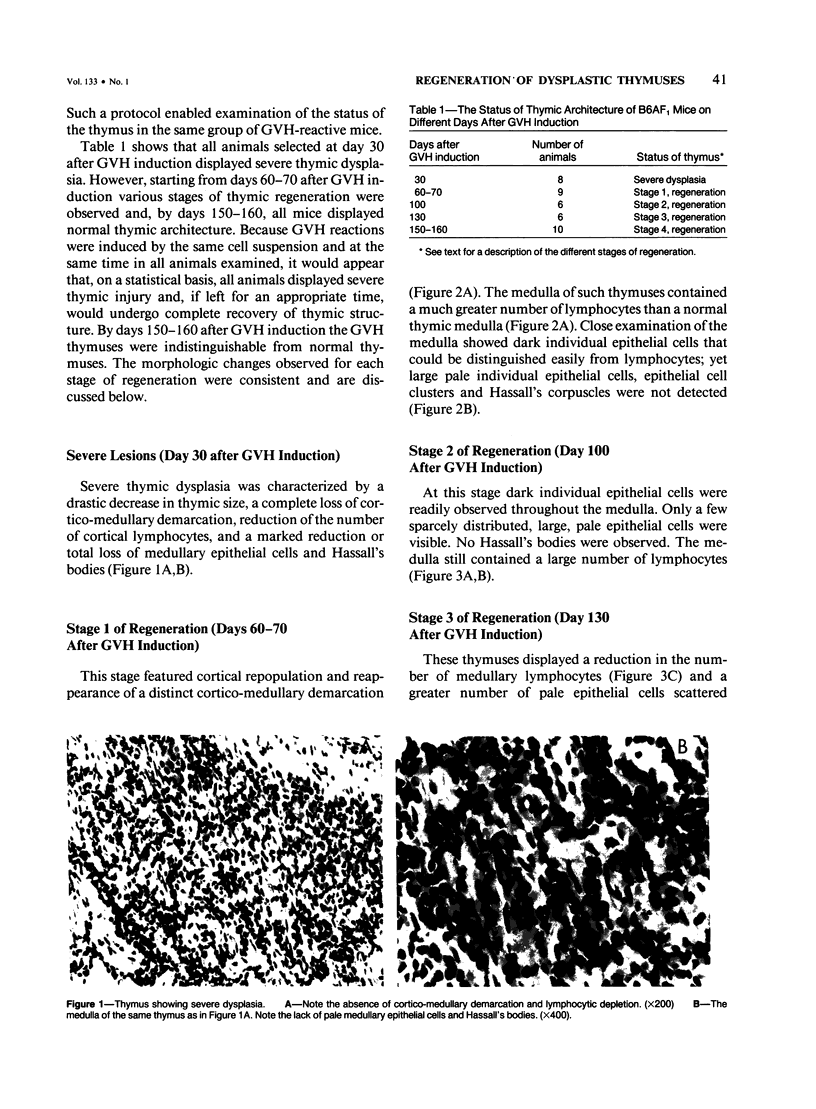
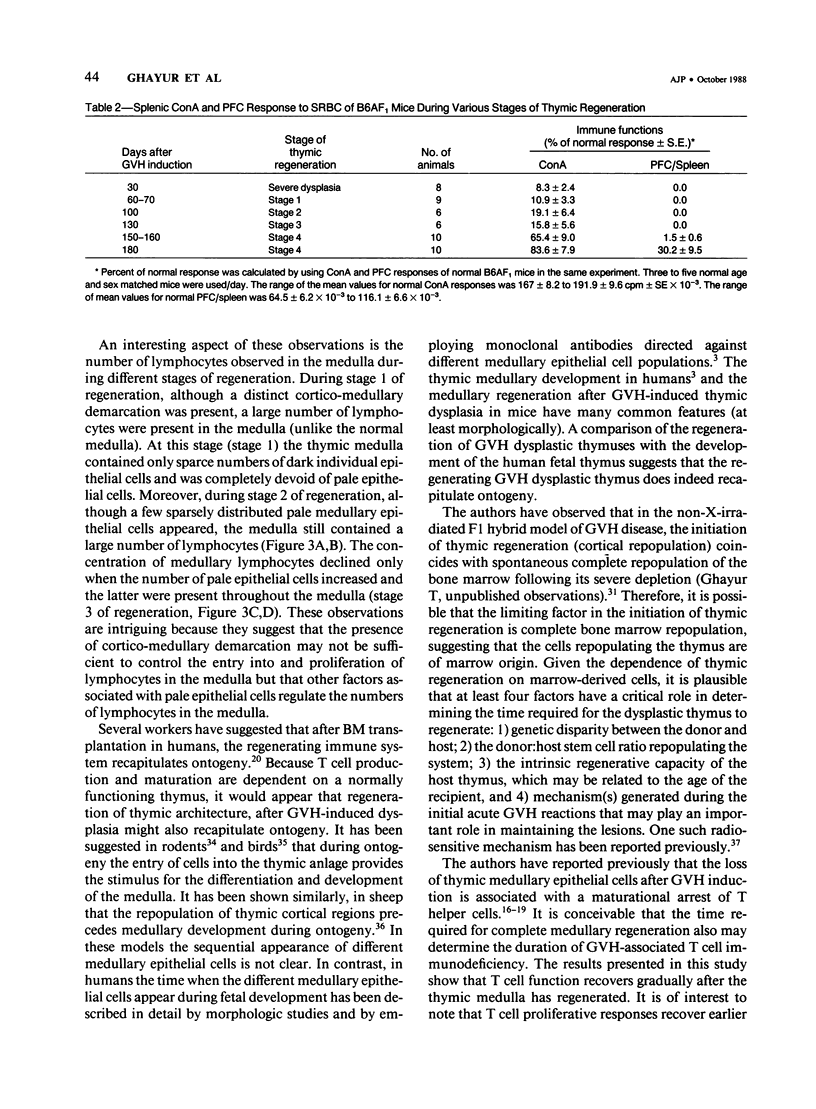
This study presents the sequential morphologic regeneration of graft-vs.-host (GVH)-induced dysplastic thymuses in long-term survivors of GVH reactions. GVH reactions were induced in adult C57BL/6xAF1 (B6AF1) hybrids by injecting 20 x 10(6) A strain parental lymphoid cells (PLC). Starting on day 30 after GVH induction, five to ten animals were randomly selected from a pool of GVH-reactive mice and killed at various times. Each animal was tested for thymic histology and T cell functions. Thymuses taken on day 30 after GVH induction displayed severe dysplasia as characterized by lymphocytic depletion, complete effacement of cortico-medullary demarcation, and reduction and total loss of medullary epithelial cells or both. Starting by days 60-70 after GVH induction, at least four stages of thymic regeneration were identified. Day 60-70 thymuses displayed cortical regeneration and the reappearance of cortico-medullary demarcation. The medulla of these thymuses, although containing dark individual epithelial cells and numerous lymphocytes, was devoid of pale epithelial cells (stage 1). The medulla of thymuses on day 100 after GVH induction displayed a few sparcely distributed pale epithelial cells and numerous lymphocytes as well as dark epithelial cells (stage 2). The medulla of thymuses examined 130 days after GVH induction displayed numerous pale individual epithelial cells and a few pale epithelial cell clusters. Such thymuses also showed a reduction in the number of medullary lymphocytes (stage 3). Finally, the medulla of thymuses 150-160 days after GVH induction displayed numerous pale epithelial cell clusters and Hassall's bodies. These thymuses were indistinguishable from normal adult thymuses (stage 4). All of the animals tested up to day 130 after GVH induction showed no significant T cell function. Animals displaying stage 4 of thymic regeneration showed significant proliferative responses to T cell mitogen, concanavalin A (conA), and six of ten animals also displayed a few plaque forming cells (PFC) to sheep red blood cells (SRBC) in their spleens. Furthermore, all animals (10 of 10) killed on day 180 after GVH induction displayed significant T cell functions.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson K., Incefy G. S., Storb R., Sullivan K. M., Iwata T., Dardenne M., Ochs H. D., Good R. A., Thomas E. D. Low serum thymic hormone levels in patients with chronic graft-versus-host disease. Blood. 1982 May;59(5):1073–1077. [PubMed] [Google Scholar]
- Beschorner W. E., Hutchins G. M., Elfenbein G. J., Santos G. W. The thymus in patients with allogeneic bone marrow transplants. Am J Pathol. 1978 Jul;92(1):173–186. [PMC free article] [PubMed] [Google Scholar]
- Beschorner W. E., Namnoum J. D., Hess A. D., Shinn C. A., Santos G. W. Cyclosporin A and the thymus. Immunopathology. Am J Pathol. 1987 Mar;126(3):487–496. [PMC free article] [PubMed] [Google Scholar]
- Beschorner W. E., Tutschka P. J., Santos G. W. Chronic graft-versus-host disease in the rat radiation chimera. I. Clinical features, hematology, histology, and immunopathology in long-term chimeras. Transplantation. 1982 Apr;33(4):393–399. doi: 10.1097/00007890-198204000-00010. [DOI] [PubMed] [Google Scholar]
- Beschorner W. E., Tutschka P. J., Santos G. W. Sequential morphology of graft-versus-host disease in the rat radiation chimera. Clin Immunol Immunopathol. 1982 Feb;22(2):203–224. doi: 10.1016/0090-1229(82)90038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich W., O'Reilly R. J., Koziner B., Gebhard D. F., Jr, Good R. A., Evans R. L. T-lymphocyte reconstitution in recipients of bone marrow transplants with and without GVHD: imbalances of T-cell subpopulations having unique regulatory and cognitive functions. Blood. 1982 Apr;59(4):696–701. [PubMed] [Google Scholar]
- Gale R. P. Graft-versus-host disease. Immunol Rev. 1985 Dec;88:193–214. doi: 10.1111/j.1600-065x.1985.tb01159.x. [DOI] [PubMed] [Google Scholar]
- Haynes B. F. The human thymic microenvironment. Adv Immunol. 1984;36:87–142. doi: 10.1016/s0065-2776(08)60900-1. [DOI] [PubMed] [Google Scholar]
- Hess A. D., Horwitz L., Beschorner W. E., Santos G. W. Development of graft-vs.-host disease-like syndrome in cyclosporine-treated rats after syngeneic bone marrow transplantation. I. Development of cytotoxic T lymphocytes with apparent polyclonal anti-Ia specificity, including autoreactivity. J Exp Med. 1985 Apr 1;161(4):718–730. doi: 10.1084/jem.161.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M., Jotereau F. V. Tracing of cells of the avian thymus through embryonic life in interspecific chimeras. J Exp Med. 1975 Jul 1;142(1):17–40. doi: 10.1084/jem.142.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum L. G., Orcutt-Thordarson N., Seigneuret M. C., Storb R. The regulation of Ig synthesis after marrow transplantation. IV. T4 and T8 subset function in patients with chronic graft-vs-host disease. J Immunol. 1982 Jul;129(1):113–119. [PubMed] [Google Scholar]
- Mackay C. R., Maddox J. F., Brandon M. R. Thymocyte subpopulations during early fetal development in sheep. J Immunol. 1986 Mar 1;136(5):1592–1599. [PubMed] [Google Scholar]
- Martin P. J., Hansen J. A., Storb R., Thomas E. D. Human marrow transplantation: an immunological perspective. Adv Immunol. 1987;40:379–438. doi: 10.1016/s0065-2776(08)60243-6. [DOI] [PubMed] [Google Scholar]
- Mendes M. L., Rode H., Peres A., Kongshavn P. A., Lapp W. S. Interleukin-1 and interleukin-2 defects associated with murine graft-versus-host-induced immunodeficiency. Transplantation. 1985 Apr;39(4):418–424. doi: 10.1097/00007890-198504000-00016. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Owen J. J. Experimental studies on the development of the thymus. J Exp Med. 1967 Oct 1;126(4):715–726. doi: 10.1084/jem.126.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Hermelink H. K., Sale G. E., Borisch B., Storb R. Pathology of the thymus after allogeneic bone marrow transplantation in man. A histologic immunohistochemical study of 36 patients. Am J Pathol. 1987 Nov;129(2):242–256. [PMC free article] [PubMed] [Google Scholar]
- Müller-Ruchholtz W., Wottge H. U., Müller-Hermelink H. K. Restitution potentials of allogeneically or xenogeneically grafted lymphocyte-free hemopoietic stem cells. Haematol Blood Transfus. 1980;25:153–177. doi: 10.1007/978-3-642-67319-1_15. [DOI] [PubMed] [Google Scholar]
- Noel D. R., Witherspoon R. P., Storb R., Atkinson K., Doney K., Mickelson E. M., Ochs H. D., Warren R. P., Weiden P. L., Thomas E. D. Does graft-versus-host disease influence the tempo of immunologic recovery after allogeneic human marrow transplantation? An observation on 56 long-term survivors. Blood. 1978 Jun;51(6):1087–1105. [PubMed] [Google Scholar]
- Parkman R. Clonal analysis of murine graft-vs-host disease. I. Phenotypic and functional analysis of T lymphocyte clones. J Immunol. 1986 May 15;136(10):3543–3548. [PubMed] [Google Scholar]
- Rappaport H., Khalil A., Halle-Pannenko O., Pritchard L., Dantchev D., Mathé G. Histopathologic sequence of events in adult mice undergoing lethal graft-versus-host reaction developed across H-2 and/or non-H-2 histocompatibility barriers. Am J Pathol. 1979 Jul;96(1):121–142. [PMC free article] [PubMed] [Google Scholar]
- Santos G. W., Hess A. D., Vogelsang G. B. Graft-versus-host reactions and disease. Immunol Rev. 1985 Dec;88:169–192. doi: 10.1111/j.1600-065x.1985.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Schroff R. W., Gale R. P., Fahey J. L. Regeneration of T cell subpopulations after bone marrow transplantation: cytomegalovirus infection and lymphoid subset imbalance. J Immunol. 1982 Nov;129(5):1926–1930. [PubMed] [Google Scholar]
- Seddik M., Seemayer T. A., Lapp W. S. T cell functional defect associated with thymid epithelial cell injury induced by a graft-versus-host reaction. Transplantation. 1980;29(1):61–66. doi: 10.1097/00007890-198001000-00013. [DOI] [PubMed] [Google Scholar]
- Seddik M., Seemayer T. A., Lapp W. S. The graft-versus-host reaction and immune function. I. T helper cell immunodeficiency associated with graft-versus-host-induced thymic epithelial cell damage. Transplantation. 1984 Mar;37(3):281–286. doi: 10.1097/00007890-198403000-00013. [DOI] [PubMed] [Google Scholar]
- Seddik M., Seemayer T. A., Lapp W. S. The graft-versus-host reaction and immune function. II. Recruitment of pre-T-cells in vivo by graft-versus-host-induced dysplastic thymuses following irradiation and bone marrow treatment. Transplantation. 1984 Mar;37(3):286–290. [PubMed] [Google Scholar]
- Seemayer T. A., Lapp W. S., Bolande R. P. Thymic epithelial injury in graft-versus-host reactions following adrenalectomy. Am J Pathol. 1978 Nov;93(2):325–338. [PMC free article] [PubMed] [Google Scholar]
- Seemayer T. A., Lapp W. S., Bolande R. P. Thymic involution in murine graft-versus-host reaction. Epithelial injury mimicking human thymic dysplasia. Am J Pathol. 1977 Jul;88(1):119–134. [PMC free article] [PubMed] [Google Scholar]
- Seemayer T. A., Laroche A. C., Russo P., Malebranche R., Arnoux E., Guérin J. M., Pierre G., Dupuy J. M., Gartner J. G., Lapp W. S. Precocious thymic involution manifest by epithelial injury in the acquired immune deficiency syndrome. Hum Pathol. 1984 May;15(5):469–474. doi: 10.1016/s0046-8177(84)80082-9. [DOI] [PubMed] [Google Scholar]
- Seemayer T. A. The Graft-versus-Host Reaction: a pathogenetic mechanism of experimental and human disease. Perspect Pediatr Pathol. 1979;5:93–136. [PubMed] [Google Scholar]
- Stutman O. Intrathymic and extrathymic T cell maturation. Immunol Rev. 1978;42:138–184. doi: 10.1111/j.1600-065x.1978.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Sloane J. P., Imrie S. F., Ritter M. A., Schuurman H. J., Huber J. Immunohistology of the thymus in bone marrow transplant recipients. Am J Pathol. 1986 Mar;122(3):531–540. [PMC free article] [PubMed] [Google Scholar]
- Witherspoon R. P., Storb R., Ochs H. D., Fluornoy N., Kopecky K. J., Sullivan K. M., Deeg J. H., Sosa R., Noel D. R., Atkinson K. Recovery of antibody production in human allogeneic marrow graft recipients: influence of time posttransplantation, the presence or absence of chronic graft-versus-host disease, and antithymocyte globulin treatment. Blood. 1981 Aug;58(2):360–368. [PubMed] [Google Scholar]
- Xenocostas A., Lapp W. S., Osmond D. G. Suppression of B lymphocyte genesis in the bone marrow by systemic graft-versus-host reactions. Transplantation. 1987 Apr;43(4):549–555. doi: 10.1097/00007890-198704000-00019. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M. Thymus and lymphohemopoietic cells: their role in T cell maturation in selection of T cells' H-2-restriction-specificity and in H-2 linked Ir gene control. Immunol Rev. 1978;42:224–270. doi: 10.1111/j.1600-065x.1978.tb00264.x. [DOI] [PubMed] [Google Scholar]
- van de Griend R. J., Astaldi A., Vossen J. M., Dooren L. J., Schellekens P. T., Zwaan F. E., van den Ende A., Roos M., Roos D. T lymphocyte characteristics in bone marrow-transplanted patients. I. Changes in biochemical properties that correlate with the immunologic reconstitution. J Immunol. 1981 Feb;126(2):636–640. [PubMed] [Google Scholar]






