Abstract
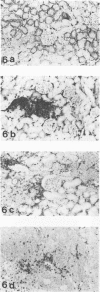
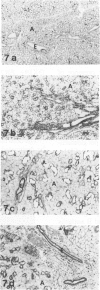
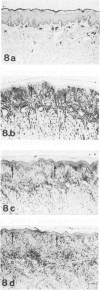
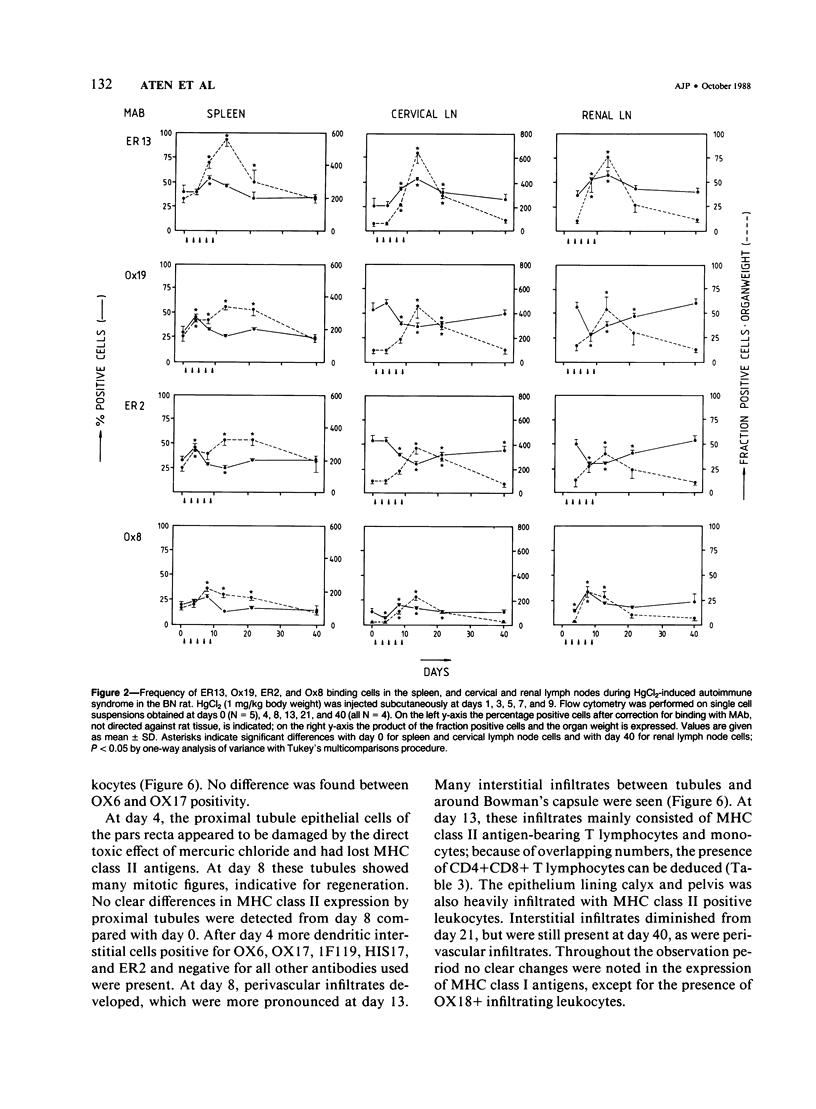
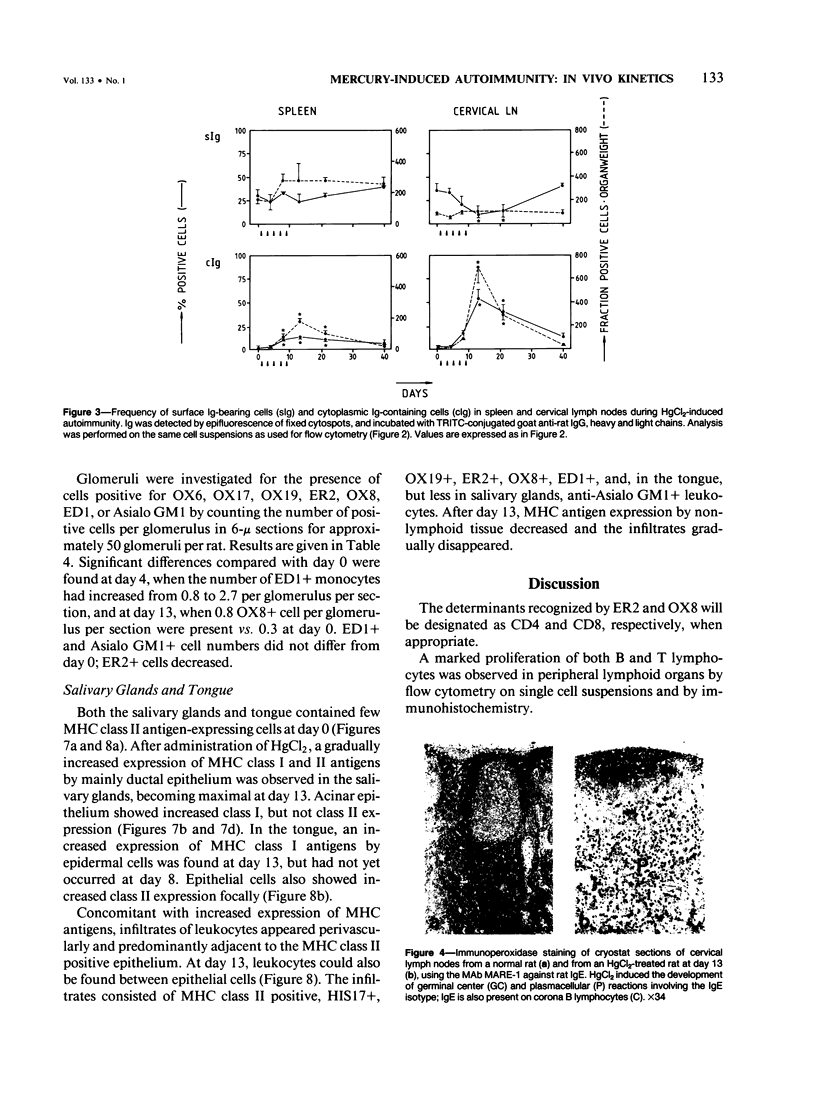
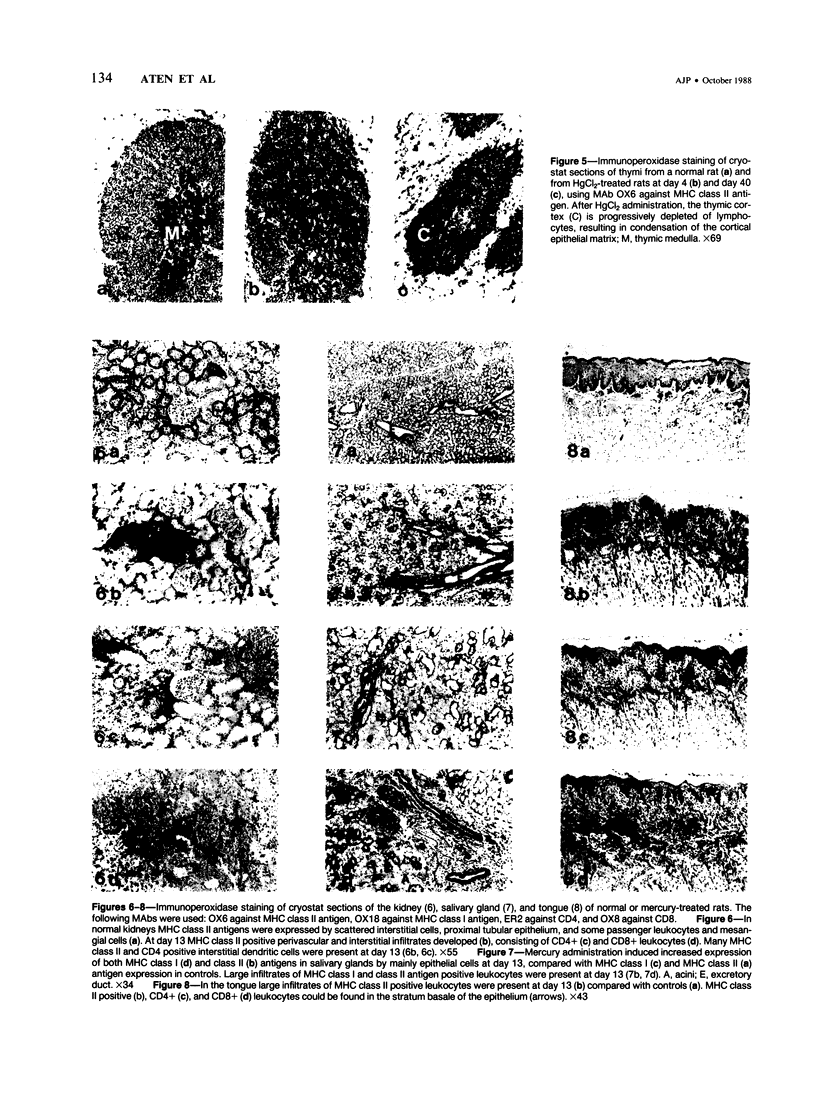
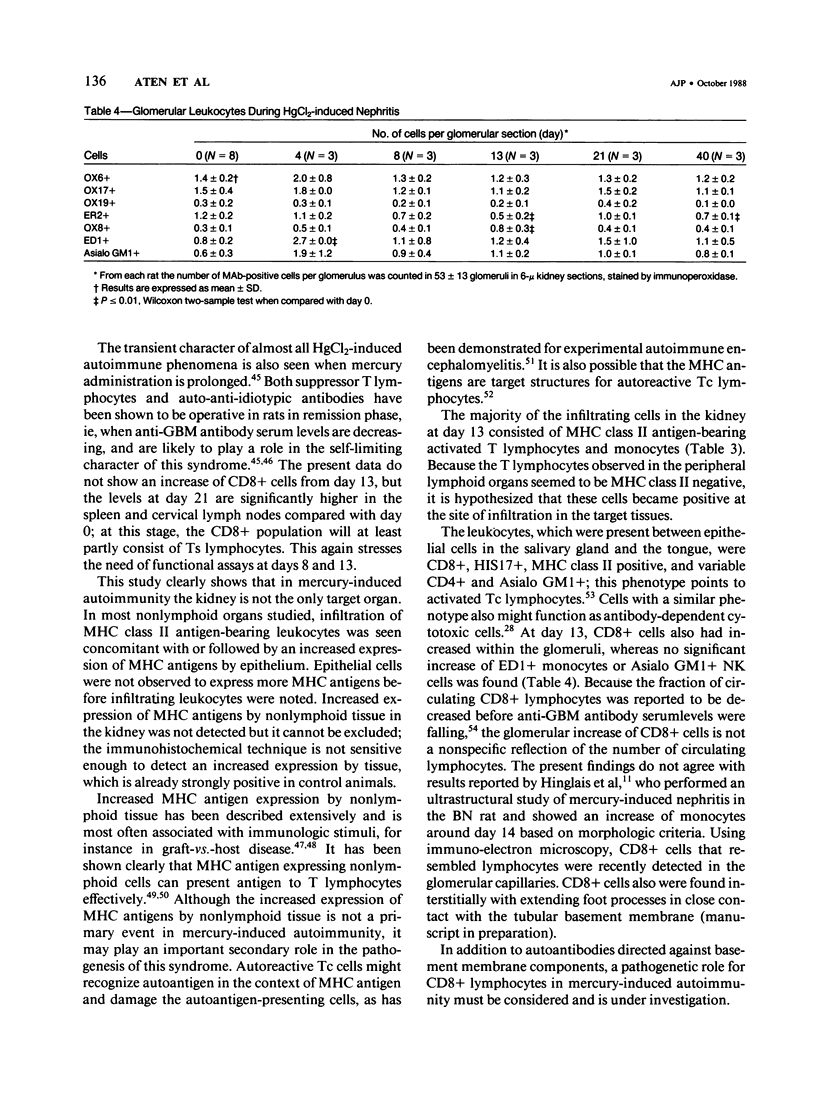
HgCl2 induces an autoimmune syndrome in Brown Norway rats that involves synthesis of anti-glomerular basement membrane (GBM) antibodies and development of nephritis with high proteinuria. HgCl2-induced changes in the composition of leukocyte populations and in the expression of MHC antigens in lymphoid and nonlymphoid organs were investigated by flow cytometry and immunohistochemistry. An early increase of CD4+ splenocytes was followed by a transient proliferation of CD4+ as well as CD8+ and B lymphocytes in peripheral lymphoid organs; in contrast, progressive depletion of the thymic cortex was found. B lymphocyte activation involved mainly the IgG1 and IgE isotypes. Nonlymphoid organs were infiltrated by MHC class II antigen expressing CD4+ and CD8+ T lymphocytes and monocytes; secondary to infiltration, mainly epithelial cells, being the main target of infiltrating cells, showed increased expression of MHC antigens. In glomeruli a 2.7-fold increase of CD8+ lymphocytes occurred after HgCl2-administration. The diverse autoimmune phenomena observed in this study fit with the hypothesized involvement of T lymphocytes autoreactive with MHC class II antigens. Apart from anti-GBM autoantibodies, a role for autoreactive CD8+ T lymphocytes must be considered in the pathogenesis of the HgCl2-induced autoimmune syndrome.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baran D., Vendeville B., Vial M. C., Cosson C., Bascou C., Teychenne P., Druet P. Effect of cyclosporine A on mercury-induced autoimmune glomerulonephritis in the Brown Norway rat. Clin Nephrol. 1986;25 (Suppl 1):S175–S180. [PubMed] [Google Scholar]
- Bellon B., Capron M., Druet E., Verroust P., Vial M. C., Sapin C., Girard J. F., Foidart J. M., Mahieu P., Druet P. Mercuric chloride induced autoimmune disease in Brown-Norway rats: sequential search for anti-basement membrane antibodies and circulating immune complexes. Eur J Clin Invest. 1982 Apr;12(2):127–133. doi: 10.1111/j.1365-2362.1982.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Bernaudin J. F., Druet E., Belair M. F., Pinchon M. C., Sapin C., Druet P. Extrarenal immune complex type deposits induced by mercuric chloride in the Brown Norway rat. Clin Exp Immunol. 1979 Nov;38(2):265–273. [PMC free article] [PubMed] [Google Scholar]
- Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Bowman C., Green C., Borysiewicz L., Lockwood C. M. Circulating T-cell populations during mercuric chloride-induced nephritis in the Brown Norway rat. Immunology. 1987 Aug;61(4):515–520. [PMC free article] [PubMed] [Google Scholar]
- Bowman C., Mason D. W., Pusey C. D., Lockwood C. M. Autoregulation of autoantibody synthesis in mercuric chloride nephritis in the Brown Norway rat. I. A role for T suppressor cells. Eur J Immunol. 1984 May;14(5):464–470. doi: 10.1002/eji.1830140515. [DOI] [PubMed] [Google Scholar]
- Bowman C., Peters D. K., Lockwood C. M. Anti-glomerular basement membrane autoantibodies in the Brown Norway rat: detection by a solid-phase radioimmunoassay. J Immunol Methods. 1983 Jul 29;61(3):325–333. doi: 10.1016/0022-1759(83)90227-2. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Robins R. A., Brooks C. G., Baldwin R. W. Phenotype of rat natural killer cells defined by monoclonal antibodies marking rat lymphocyte subsets. Immunology. 1982 Jan;45(1):97–103. [PMC free article] [PubMed] [Google Scholar]
- Capron M., Bascou C., Vial M. C., Grossetete J., Hinglais N., Girard J. F., Druet P. Effects of decomplementation on mercuric chloride-induced glomerulonephritis in Brown-Norway rats. Clin Exp Immunol. 1982 Sep;49(3):611–617. [PMC free article] [PubMed] [Google Scholar]
- Chalopin J. M., Lockwood C. M. Autoregulation of autoantibody synthesis in mercuric chloride nephritis in the Brown Norway rat. II. Presence of antigen-augmentable plaque-forming cells in the spleen is associated with humoral factors behaving as auto-anti-idiotypic antibodies. Eur J Immunol. 1984 May;14(5):470–475. doi: 10.1002/eji.1830140516. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Thomas M. L., Green J. R. MRC OX-19: a monoclonal antibody that labels rat T lymphocytes and augments in vitro proliferative responses. Eur J Immunol. 1984 Mar;14(3):260–267. doi: 10.1002/eji.1830140311. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Druet E., Sapin C., Günther E., Feingold N., Druet P. Mercuric chloride-induced anti-glomerular basement membrane antibodies in the rat: genetic control. Eur J Immunol. 1977 Jun;7(6):348–351. doi: 10.1002/eji.1830070605. [DOI] [PubMed] [Google Scholar]
- Duarte A. J., Carpenter C. B., Strom T. B. Expression of T cell differentiation antigens and Ia on rat cytotoxic T lymphocytes. J Immunol. 1982 Feb;128(2):580–584. [PubMed] [Google Scholar]
- Eddy A. A., Crary G. S., Michael A. F. Identification of lymphohemopoietic cells in the kidneys of normal rats. Am J Pathol. 1986 Aug;124(2):335–342. [PMC free article] [PubMed] [Google Scholar]
- Finnegan A., Needleman B., Hodes R. J. Activation of B cells by autoreactive T cells: cloned autoreactive T cells activate B cells by two distinct pathways. J Immunol. 1984 Jul;133(1):78–85. [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Fukumoto T., McMaster W. R., Williams A. F. Mouse monoclonal antibodies against rat major histocompatibility antigens. Two Ia antigens and expression of Ia and class I antigens in rat thymus. Eur J Immunol. 1982 Mar;12(3):237–243. doi: 10.1002/eji.1830120313. [DOI] [PubMed] [Google Scholar]
- Habu S., Fukui H., Shimamura K., Kasai M., Nagai Y., Okumura K., Tamaoki N. In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J Immunol. 1981 Jul;127(1):34–38. [PubMed] [Google Scholar]
- Hess A. D., Horwitz L., Beschorner W. E., Santos G. W. Development of graft-vs.-host disease-like syndrome in cyclosporine-treated rats after syngeneic bone marrow transplantation. I. Development of cytotoxic T lymphocytes with apparent polyclonal anti-Ia specificity, including autoreactivity. J Exp Med. 1985 Apr 1;161(4):718–730. doi: 10.1084/jem.161.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinglais N., Druet P., Grossetete J., Sapin C., Bariety J. Ultrastructural study of nephritis induced in Brown Norway rats by mercuric chloride. Lab Invest. 1979 Aug;41(2):150–159. [PubMed] [Google Scholar]
- Hirsch F., Couderc J., Sapin C., Fournie G., Druet P. Polyclonal effect of HgCl2 in the rat, its possible role in an experimental autoimmune disease. Eur J Immunol. 1982 Jul;12(7):620–625. doi: 10.1002/eji.1830120716. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Davidson R. S., McNamee K. C., Russell G., Goodwin D., Holborow E. J. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982 Dec 17;55(2):231–242. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Kelly C. J., Silvers W. K., Neilson E. G. Tolerance to parenchymal self. Regulatory role of major histocompatibility complex-restricted, OX8+ suppressor T cells specific for autologous renal tubular antigen in experimental interstitial nephritis. J Exp Med. 1985 Dec 1;162(6):1892–1903. doi: 10.1084/jem.162.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese F. G., Opstelten D., Wubbena A. S., Deenen G. J., Aten J., Schwander E. H., de Leij L., Nieuwenhuis P. Monoclonal antibodies to rat B lymphocyte (sub-)populations. Adv Exp Med Biol. 1985;186:81–89. doi: 10.1007/978-1-4613-2463-8_10. [DOI] [PubMed] [Google Scholar]
- Kroese F. G., Wubbena A. S., Joling P., Nieuwenhuis P. T-lymphocytes in rat lymphoid follicles are a subset of T helper cells. Adv Exp Med Biol. 1985;186:443–449. doi: 10.1007/978-1-4613-2463-8_54. [DOI] [PubMed] [Google Scholar]
- Leung D. Y., Young M. C., Geha R. S. Induction of IgG and IgE synthesis in normal B cells by autoreactive T cell clones. J Immunol. 1986 Apr 15;136(8):2851–2855. [PubMed] [Google Scholar]
- Londei M., Lamb J. R., Bottazzo G. F., Feldmann M. Epithelial cells expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 1984 Dec 13;312(5995):639–641. doi: 10.1038/312639a0. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Dallman M., Barclay A. N. Graft-versus-host disease induces expression of Ia antigen in rat epidermal cells and gut epithelium. Nature. 1981 Sep 10;293(5828):150–151. doi: 10.1038/293150a0. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Nagarkatti P. S., Nagarkatti M., Mann L. W., Jones L. A., Kaplan A. M. Characterization of an endogenous Lyt2+ T-suppressor-cell population regulating autoreactive T cells in vitro and in vivo. Cell Immunol. 1988 Mar;112(1):64–77. doi: 10.1016/0008-8749(88)90276-6. [DOI] [PubMed] [Google Scholar]
- Opstelten D., Deenen G. J., Rozing J., Hunt S. V. B lymphocyte-associated antigens on terminal deoxynucleotidyl transferase-positive cells and pre-B cells in bone marrow of the rat. J Immunol. 1986 Jul 1;137(1):76–84. [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Hirsch F., Sapin C., Druet P. Autoreactive T cells in mercury-induced autoimmune disease: in vitro demonstration. J Immunol. 1986 Oct 15;137(8):2548–2554. [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Rossert J., Vial M. C., Mandet C., Druet P. Autoreactive T cells in mercury-induced autoimmunity. Ability to induce the autoimmune disease. J Immunol. 1988 Feb 1;140(3):750–754. [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Vial M. C., Mandet C., Moutier R., Salomon J. C., Druet P. Mercury-induced autoimmune glomerulonephritis: requirement for T-cells. Nephrol Dial Transplant. 1987;1(4):211–218. [PubMed] [Google Scholar]
- Prouvost-Danon A., Abadie A., Sapin C., Bazin H., Druet P. Induction of IgE synthesis and potentiation of anti-ovalbumin IgE antibody response by HgCl2 in the rat. J Immunol. 1981 Feb;126(2):699–792. [PubMed] [Google Scholar]
- Reynolds C. W., Sharrow S. O., Ortaldo J. R., Herberman R. B. Natural killer activity in the rat. II. Analysis of surface antigens on LGL by flow cytometry. J Immunol. 1981 Dec;127(6):2204–2208. [PubMed] [Google Scholar]
- Saito T., Rajewsky K. A self-Ia reactive T cell clone directly stimulates every hundredth B cell and helps antigen-specific B cell responses. Eur J Immunol. 1985 Sep;15(9):927–934. doi: 10.1002/eji.1830150912. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Fukuma K., Kuribayashi K., Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985 Jan 1;161(1):72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N. Thymus and autoimmunity. Transplantation of the thymus from cyclosporin A-treated mice causes organ-specific autoimmune disease in athymic nude mice. J Exp Med. 1988 Apr 1;167(4):1479–1485. doi: 10.1084/jem.167.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapin C., Druet E., Druet P. Induction of anti-glomerular basement membrane antibodies in the Brown-Norway rat by mercuric chloride. Clin Exp Immunol. 1977 Apr;28(1):173–179. [PMC free article] [PubMed] [Google Scholar]
- Stet R. J., Thomas C., Koudstaal J., Hardonk M. J., Hulstaert C. E., Nieuwenhuis P. Graft-versus-host disease in the rat: cellular changes and major histocompatibility complex antigen expression in the liver. Scand J Immunol. 1986 Jan;23(1):81–89. doi: 10.1111/j.1365-3083.1986.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Sun D., Wekerle H. Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature. 1986 Mar 6;320(6057):70–72. doi: 10.1038/320070a0. [DOI] [PubMed] [Google Scholar]
- Tomonari K. In vivo helper activity of autoreactive T cell clones. J Immunol. 1985 Sep;135(3):1598–1602. [PubMed] [Google Scholar]
- Weening J. J., Fleuren G. J., Hoedemaeker P. J. Demonstration of antinuclear antibodies in mercuric chloride-induced glomerulopathy in the rat. Lab Invest. 1978 Oct;39(4):405–411. [PubMed] [Google Scholar]
- Weening J. J., Hoedemaeker P. J., Bakker W. W. Immunoregulation and anti-nuclear antibodies in mercury-induced glomerulopathy in the rat. Clin Exp Immunol. 1981 Jul;45(1):64–71. [PMC free article] [PubMed] [Google Scholar]
- de Heer E., Daha M. R., Burgers J., van Es L. A. Reestablishment of self tolerance by suppressor T-cells after active Heymann's nephritis. Cell Immunol. 1986 Mar;98(1):28–33. doi: 10.1016/0008-8749(86)90264-9. [DOI] [PubMed] [Google Scholar]