Abstract
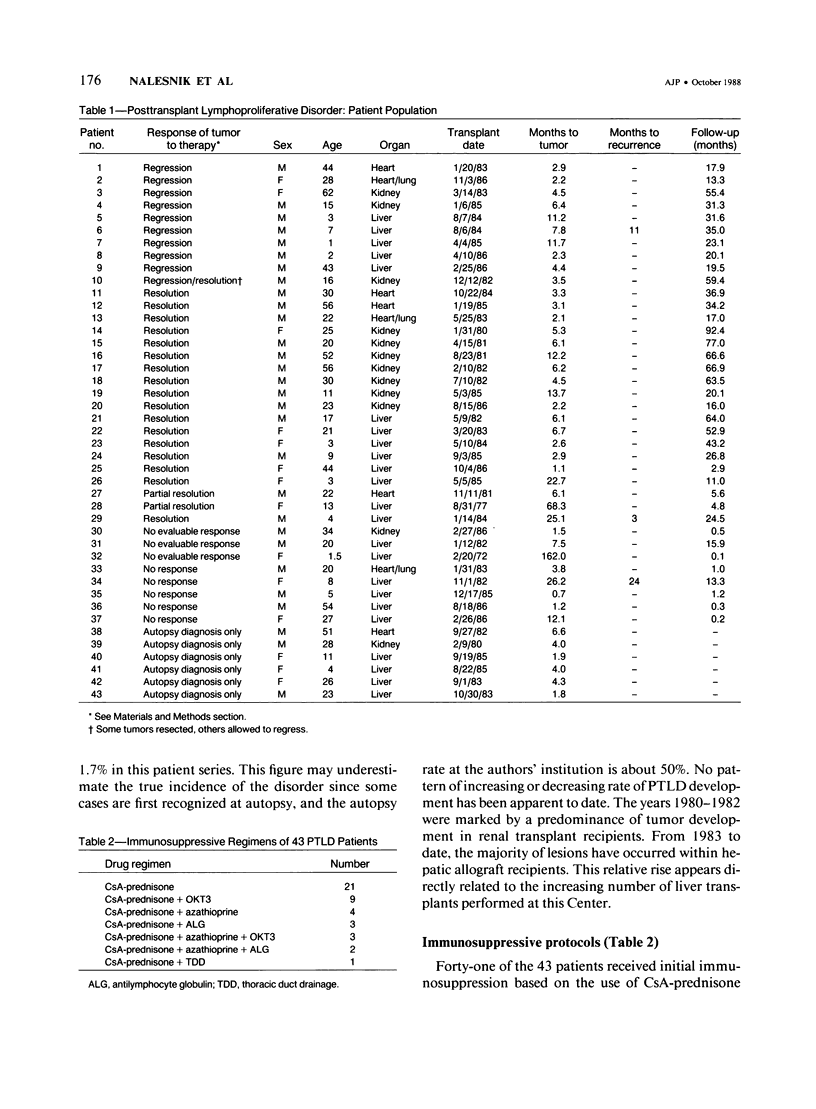
Posttransplant lymphoproliferative disorders (PTLDs) were diagnosed in 43 patients from the Pittsburgh-Denver series between June 1980 and March 1987. This constitutes a detection rate of 1.7%. Major categories of clinical presentation included a mononucleosislike syndrome, gastrointestinal/abdominal disease, and solid organ disease. The median time of onset in patients initially immunosuppressed with cyclosporine-A (CsA)-containing regimens was 4.4 months after transplant, regardless of tumor clonality. A strong association of PTLD with Epstein-Barr virus (EBV) was observed. A histologic spectrum of lesions from polymorphic to monomorphic was observed. Whereas polymorphic lesions could be either clonal or nonclonal, monomorphic lesions appeared to be clonal in composition. The presence of large atypical cells (atypical immunoblasts) or necrosis did not appreciably worsen the prognosis. Twelve patients had clonal, 13 had nonclonal, and five had both clonal and nonclonal tumors. Clonality was indeterminate in 13 cases. Most patients were treated with a regimen based on reduced immunosuppression and supportive surgery. Almost all nonclonal and about half of the clonal lesions respond to this conservative therapy, indicating that it is an appropriate first line of treatment. This behavior suggests that a spectrum of lesions ranging from infectious mononucleosis to malignant lymphoma constitutes the entity known as PTLD. Some monoclonal tumors can undergo regression, however, apparently in response to host immune control mechanisms. Because of its short latency and strong association with EBV, PTLD is an important model for the study of virus-associated tumor progression in humans.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown N., Smith D., Miller G., Niederman J., Liu C., Robinson J. Infectious mononucleosis: a polyclonal B cell transformation in vivo. J Infect Dis. 1984 Oct;150(4):517–522. doi: 10.1093/infdis/150.4.517. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Lymphoproliferative disorders in cardiac transplant recipients are multiclonal lymphomas. Lancet. 1984 Sep 1;2(8401):489–493. doi: 10.1016/s0140-6736(84)92566-2. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Warnke R., Sklar J. Monoclonality of lymphoproliferative lesions in cardiac-transplant recipients. Clonal analysis based on immunoglobulin-gene rearrangements. N Engl J Med. 1984 Feb 23;310(8):477–482. doi: 10.1056/NEJM198402233100801. [DOI] [PubMed] [Google Scholar]
- Frizzera G., Hanto D. W., Gajl-Peczalska K. J., Rosai J., McKenna R. W., Sibley R. K., Holahan K. P., Lindquist L. L. Polymorphic diffuse B-cell hyperplasias and lymphomas in renal transplant recipients. Cancer Res. 1981 Nov;41(11 Pt 1):4262–4279. [PubMed] [Google Scholar]
- Graham D. E. The isolation of high molecular weight DNA from whole organisms or large tissue masses. Anal Biochem. 1978 Apr;85(2):609–613. doi: 10.1016/0003-2697(78)90262-2. [DOI] [PubMed] [Google Scholar]
- Hanto D. W., Frizzera G., Gajl-Peczalska K. J., Sakamoto K., Purtilo D. T., Balfour H. H., Jr, Simmons R. L., Najarian J. S. Epstein-Barr virus-induced B-cell lymphoma after renal transplantation: acyclovir therapy and transition from polyclonal to monoclonal B-cell proliferation. N Engl J Med. 1982 Apr 15;306(15):913–918. doi: 10.1056/NEJM198204153061506. [DOI] [PubMed] [Google Scholar]
- Hanto D. W., Frizzera G., Gajl-Peczalska K. J., Simmons R. L. Epstein-Barr virus, immunodeficiency, and B cell lymphoproliferation. Transplantation. 1985 May;39(5):461–472. doi: 10.1097/00007890-198505000-00001. [DOI] [PubMed] [Google Scholar]
- Hanto D. W., Gajl-Peczalska K. J., Frizzera G., Arthur D. C., Balfour H. H., Jr, McClain K., Simmons R. L., Najarian J. S. Epstein-Barr virus (EBV) induced polyclonal and monoclonal B-cell lymphoproliferative diseases occurring after renal transplantation. Clinical, pathologic, and virologic findings and implications for therapy. Ann Surg. 1983 Sep;198(3):356–369. doi: 10.1097/00000658-198309000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M., Jaffe R., Miller G., Breinig M. K., Dummer J. S., Makowka L., Atchison R. W., Karrer F., Nalesnik M. A., Starzl T. E. The frequency of Epstein-Barr virus infection and associated lymphoproliferative syndrome after transplantation and its manifestations in children. Transplantation. 1988 Apr;45(4):719–727. doi: 10.1097/00007890-198804000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M., Miller G., Atchison R. W., Breinig M. K., Dummer J. S., Andiman W., Starzl T. E., Eastman R., Griffith B. P., Hardesty R. L. Epstein-Barr virus infections and DNA hybridization studies in posttransplantation lymphoma and lymphoproliferative lesions: the role of primary infection. J Infect Dis. 1985 Nov;152(5):876–886. doi: 10.1093/infdis/152.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Iwatsuki S., Geis W. P., Molnar Z., Giacchino J. L., Ing T. S., Hano J. E. Systemic lymphoblastic response to antithymocyte globulin in renal allograft recipients: an initial report. J Surg Res. 1978 May;24(5):428–434. doi: 10.1016/0022-4804(78)90039-2. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Kunnath L., Locker J. Variable methylation of the ribosomal RNA genes of the rat. Nucleic Acids Res. 1982 Jul 10;10(13):3877–3892. doi: 10.1093/nar/10.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalesnik M. A., Starzl T. E., Porter K. A., Sklar J., Cleary M. L. Genotypic analyses of cyclosporine-associated lymphoproliferations. Transplantation. 1987 Apr;43(4):592–593. doi: 10.1097/00007890-198704000-00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn I. Cancers following cyclosporine therapy. Transplantation. 1987 Jan;43(1):32–35. doi: 10.1097/00007890-198701000-00008. [DOI] [PubMed] [Google Scholar]
- Penn I. Tumors arising in organ transplant recipients. Adv Cancer Res. 1978;28:31–61. doi: 10.1016/s0065-230x(08)60645-4. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T. Defective immune surveillance in viral carcinogenesis. Lab Invest. 1984 Oct;51(4):373–385. [PubMed] [Google Scholar]
- Purtilo D. T., Tatsumi E., Manolov G., Manolova Y., Harada S., Lipscomb H., Krueger G. Epstein-Barr virus as an etiological agent in the pathogenesis of lymphoproliferative and aproliferative diseases in immune deficient patients. Int Rev Exp Pathol. 1985;27:113–183. [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Saemundsen A. K., Purtilo D. T., Sakamoto K., Sullivan J. L., Synnerholm A. C., Hanto D., Simmons R., Anvret M., Collins R., Klein G. Documentation of Epstein-Barr virus infection in immunodeficient patients with life-threatening lymphoproliferative diseases by Epstein-Barr virus complementary RNA/DNA and viral DNA/DNA hybridization. Cancer Res. 1981 Nov;41(11 Pt 1):4237–4242. [PubMed] [Google Scholar]
- Sato T. Acute Epstein-Barr virus infection and diffuse large-cell lymphoma. J Infect. 1985 May;10(3):265–267. doi: 10.1016/s0163-4453(85)92738-0. [DOI] [PubMed] [Google Scholar]
- Shearer W. T., Ritz J., Finegold M. J., Guerra I. C., Rosenblatt H. M., Lewis D. E., Pollack M. S., Taber L. H., Sumaya C. V., Grumet F. C. Epstein-Barr virus-associated B-cell proliferations of diverse clonal origins after bone marrow transplantation in a 12-year-old patient with severe combined immunodeficiency. N Engl J Med. 1985 May 2;312(18):1151–1159. doi: 10.1056/NEJM198505023121804. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Nalesnik M. A., Porter K. A., Ho M., Iwatsuki S., Griffith B. P., Rosenthal J. T., Hakala T. R., Shaw B. W., Jr, Hardesty R. L. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984 Mar 17;1(8377):583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Penn I., Halgrimson C. G. Immunosuppression and malignant neoplasms. N Engl J Med. 1970 Oct 22;283(17):934–934. doi: 10.1056/NEJM197010222831714. [DOI] [PubMed] [Google Scholar]
- Weintraub J., Warnke R. A. Lymphoma in cardiac allotransplant recipients. Clinical and histological features and immunological phenotype. Transplantation. 1982 Apr;33(4):347–351. doi: 10.1097/00007890-198204000-00002. [DOI] [PubMed] [Google Scholar]









