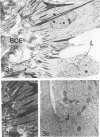
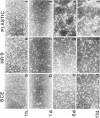
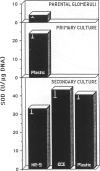
Abstract
The activities of three antioxidant enzymes, superoxide dismutase, catalase, and glutathione peroxidase, were monitored in isolated guinea pig glomeruli and primary or subcultured glomerular epithelial cells. Cell injury was assessed by morphologic studies and by measurement of cellular lipid peroxidation (levels of malondialdehyde). Antioxidant enzyme activities were very different in cultured cells than in parent glomeruli. The possible effect of culture substrates (tissue culture plastic, bovine corneal endothelial [BCE] cell basement membrane, and PF-HR-9 endodermal cell basement membrane) on antioxidant enzyme status, cell morphology, and lipid peroxidation was also assessed. Glomerular epithelial cells cultured on the BCE cell basement membrane substrate survived longer and showed less lipid peroxidation than cells cultured on plastic or the HR-9 substrate. Cells cultured on a plastic substrate had substantially less glutathione peroxidase activity than cells cultured on either BCE or HR-9 basement membranes.
Full text
PDF





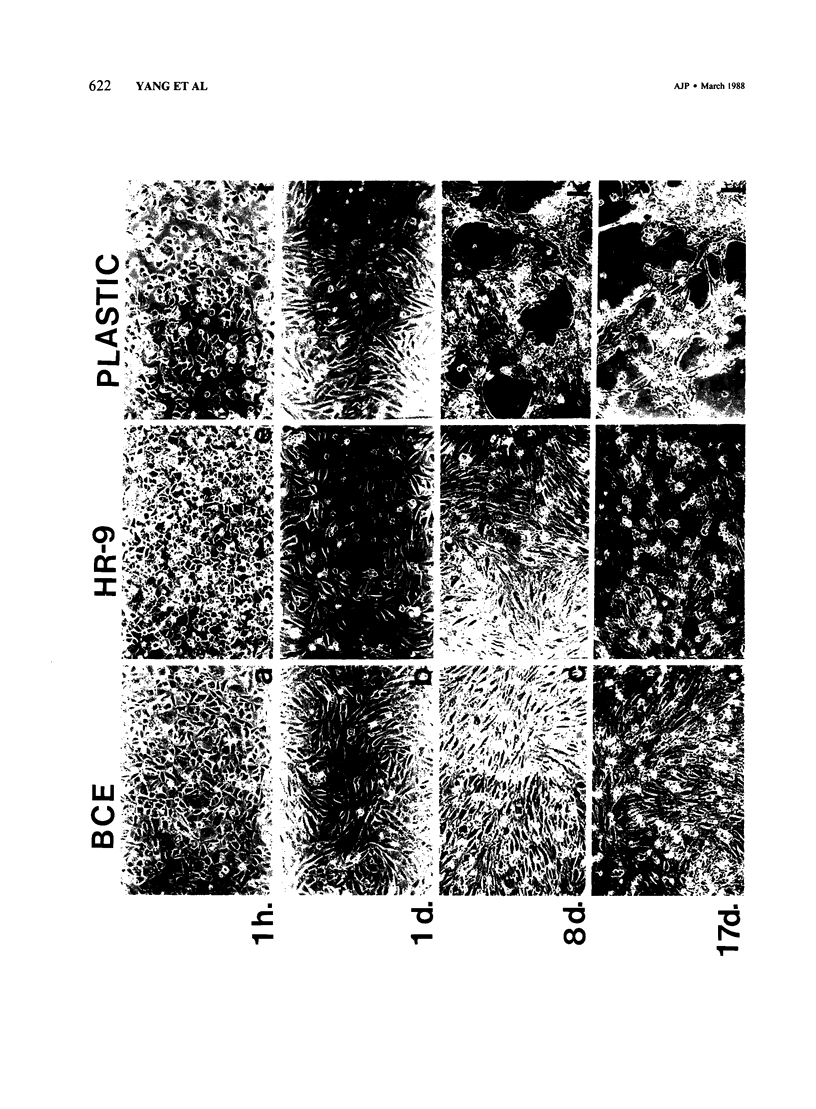
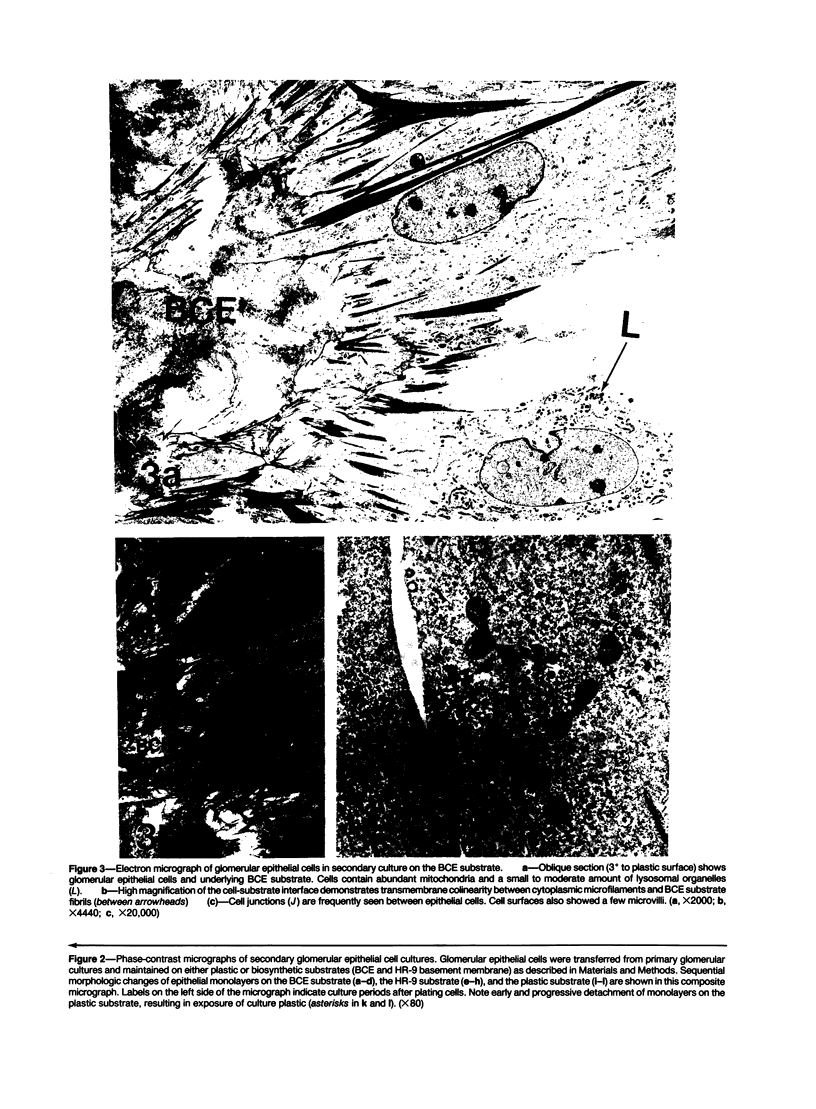
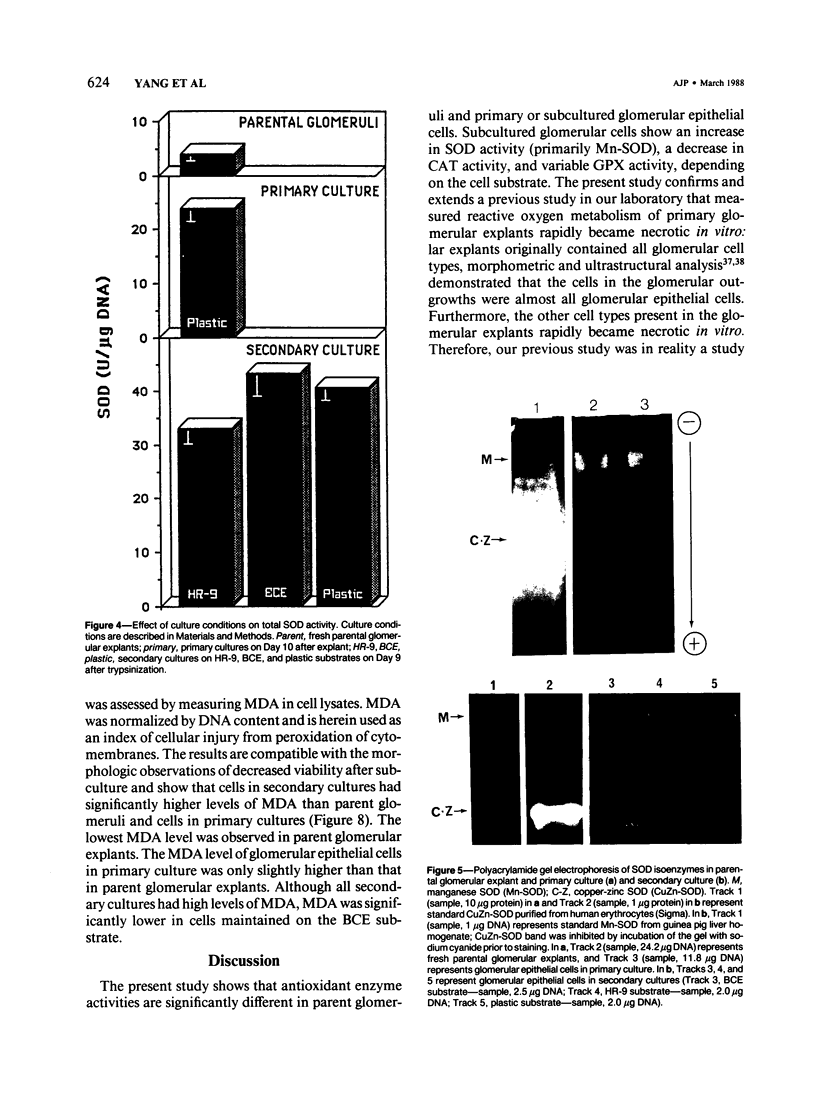

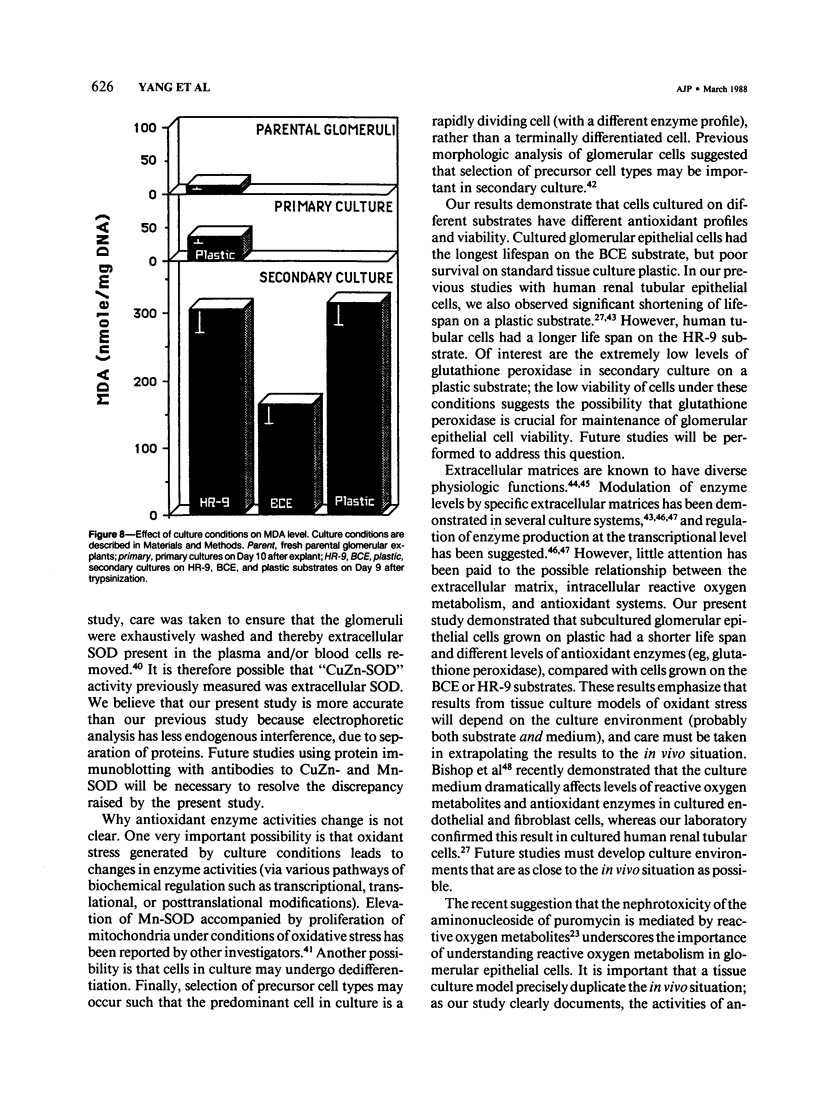


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baud L., Hagege J., Sraer J., Rondeau E., Perez J., Ardaillou R. Reactive oxygen production by cultured rat glomerular mesangial cells during phagocytosis is associated with stimulation of lipoxygenase activity. J Exp Med. 1983 Dec 1;158(6):1836–1852. doi: 10.1084/jem.158.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud L., Nivez M. P., Chansel D., Ardaillou R. Stimulation by oxygen radicals of prostaglandin production by rat renal glomeruli. Kidney Int. 1981 Sep;20(3):332–339. doi: 10.1038/ki.1981.143. [DOI] [PubMed] [Google Scholar]
- Baud L., Perez J., Ardaillou R. Dexamethasone and hydrogen peroxide production by mesangial cells during phagocytosis. Am J Physiol. 1986 Apr;250(4 Pt 2):F596–F604. doi: 10.1152/ajprenal.1986.250.4.F596. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bensinger R. E., Johnson C. M. Luminol assay for superoxide dismutase. Anal Biochem. 1981 Sep 1;116(1):142–145. doi: 10.1016/0003-2697(81)90336-5. [DOI] [PubMed] [Google Scholar]
- Bishop C. T., Mirza Z., Crapo J. D., Freeman B. A. Free radical damage to cultured porcine aortic endothelial cells and lung fibroblasts: modulation by culture conditions. In Vitro Cell Dev Biol. 1985 Apr;21(4):229–236. doi: 10.1007/BF02620934. [DOI] [PubMed] [Google Scholar]
- Brawn K., Fridovich I. Superoxide radical and superoxide dismutases: threat and defense. Acta Physiol Scand Suppl. 1980;492:9–18. [PubMed] [Google Scholar]
- Clayton D. F., Harrelson A. L., Darnell J. E., Jr Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985 Oct;5(10):2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Río L. A., Ortega M. G., López A. L., Gorgé J. L. A more sensitive modification of the catalase assay with the Clark oxygen electrode. Application to the kinetic study of the pea leaf enzyme. Anal Biochem. 1977 Jun;80(2):409–415. doi: 10.1016/0003-2697(77)90662-5. [DOI] [PubMed] [Google Scholar]
- Diamond J. R., Bonventre J. V., Karnovsky M. J. A role for oxygen free radicals in aminonucleoside nephrosis. Kidney Int. 1986 Feb;29(2):478–483. doi: 10.1038/ki.1986.24. [DOI] [PubMed] [Google Scholar]
- Downs T. R., Wilfinger W. W. Fluorometric quantification of DNA in cells and tissue. Anal Biochem. 1983 Jun;131(2):538–547. doi: 10.1016/0003-2697(83)90212-9. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Lepine J., Massoglia S., Wood I. Comparison of the ability of basement membranes produced by corneal endothelial and mouse-derived Endodermal PF-HR-9 cells to support the proliferation and differentiation of bovine kidney tubule epithelial cells in vitro. J Cell Biol. 1984 Sep;99(3):947–961. doi: 10.1083/jcb.99.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzler W. A., Kremers H., Flohé L. An improved coupled test procedure for glutathione peroxidase (EC 1-11-1-9-) in blood. Z Klin Chem Klin Biochem. 1974 Oct;12(10):444–448. doi: 10.1515/cclm.1974.12.10.444. [DOI] [PubMed] [Google Scholar]
- Lavelle F., Michelson A. M., Dimitrijevic L. Biological protection by superoxide dismutase. Biochem Biophys Res Commun. 1973 Nov 16;55(2):350–357. doi: 10.1016/0006-291x(73)91094-2. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Lee W. H., Kaetzel C. S., Parry G., Bissell M. J. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984 Sep 15;222(3):649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. P., Chignell C. F. Free radicals in pharmacology and toxicology--selected topics. Pharmacol Rev. 1981 Dec;33(4):189–211. [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Murphy P. J., Steinert B. W., Albrecht R. M. A morphologic and immunofluorescent analysis of primary guinea pig glomerular cell types grown in chemically defined media. Evidence for clonal growth and cell differentiation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;41(1-2):145–170. doi: 10.1007/BF02890278. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Steinert B. W., Anderson P. J. Kidney glomerular explants in serum-free media: role of individual medium components in cell outgrowth. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(2):161–176. doi: 10.1007/BF02899026. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Steinert B. W., Yang A. H., Anderson P. J. Kidney glomerular explants in serum-free media. Sequential morphologic and quantitative analysis of cell outgrowths. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;50(3):209–235. doi: 10.1007/BF02889903. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Yang A. H., Gould-Kostka J. Selection of kidney cell types in primary glomerular explant outgrowths by in vitro culture conditions. J Cell Sci. 1986 Aug;84:69–92. doi: 10.1242/jcs.84.1.69. [DOI] [PubMed] [Google Scholar]
- Olivetti G., Anversa P., Melissari M., Loud A. V. Morphometry of the renal corpuscle during postnatal growth and compensatory hypertrophy. Kidney Int. 1980 Apr;17(4):438–454. doi: 10.1038/ki.1980.52. [DOI] [PubMed] [Google Scholar]
- Rehan A., Johnson K. J., Kunkel R. G., Wiggins R. C. Role of oxygen radicals in phorbol myristate acetate-induced glomerular injury. Kidney Int. 1985 Mar;27(3):503–511. doi: 10.1038/ki.1985.39. [DOI] [PubMed] [Google Scholar]
- Shah S. V. Effect of enzymatically generated reactive oxygen metabolites on the cyclic nucleotide content in isolated rat glomeruli. J Clin Invest. 1984 Aug;74(2):393–401. doi: 10.1172/JCI111434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. V. Light emission by isolated rat glomeruli in response to phorbol myristate acetate. J Lab Clin Med. 1981 Jul;98(1):46–57. [PubMed] [Google Scholar]
- Sies H., Cadenas E. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci. 1985 Dec 17;311(1152):617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- Steinert B. W., Anderson P. J., Oberley L. W., Oberley T. D. Kidney glomerular explants in serum-free media: demonstration of intracellular antioxidant enzymes and active oxygen metabolites. In Vitro Cell Dev Biol. 1986 May;22(5):285–294. doi: 10.1007/BF02621232. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Akiyama S. K., Hasegawa T., Hasegawa E., Humphries M. J., Kennedy D. W., Nagata K., Urushihara H., Olden K., Chen W. T. Recent advances in research on fibronectin and other cell attachment proteins. J Cell Biochem. 1985;28(2):79–97. doi: 10.1002/jcb.240280202. [DOI] [PubMed] [Google Scholar]
- Yang A. H., Gould-Kostka J., Oberley T. D. In vitro growth and differentiation of human kidney tubular cells on a basement membrane substrate. In Vitro Cell Dev Biol. 1987 Jan;23(1):34–46. doi: 10.1007/BF02623491. [DOI] [PubMed] [Google Scholar]
- Yang A. H., Oberley T. D., Oberley L. W., Schmid S. M., Cummings K. B. In vitro modulation of antioxidant enzymes in normal and malignant renal epithelium. In Vitro Cell Dev Biol. 1987 Aug;23(8):546–558. doi: 10.1007/BF02620972. [DOI] [PubMed] [Google Scholar]
- van der Valk P., Gille J. J., Oostra A. B., Roubos E. W., Sminia T., Joenje H. Characterization of an oxygen-tolerant cell line derived from Chinese hamster ovary. Antioxygenic enzyme levels and ultrastructural morphometry of peroxisomes and mitochondria. Cell Tissue Res. 1985;239(1):61–68. doi: 10.1007/BF00214903. [DOI] [PubMed] [Google Scholar]