Abstract
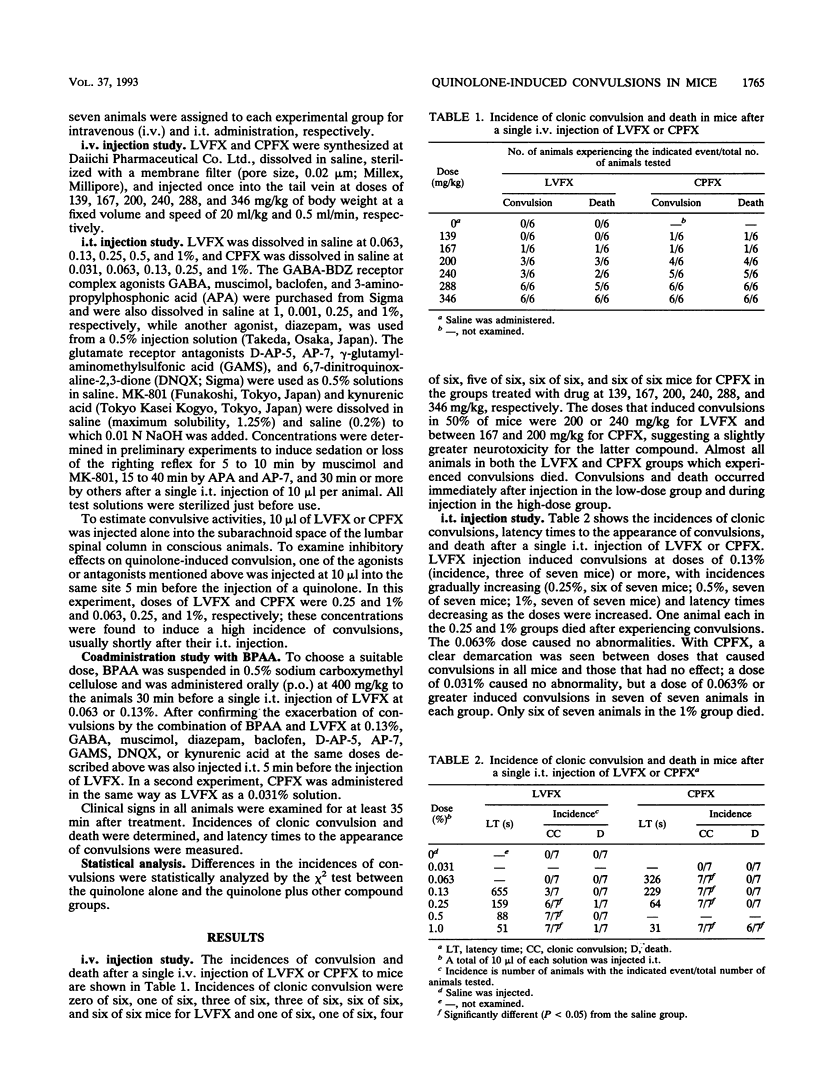
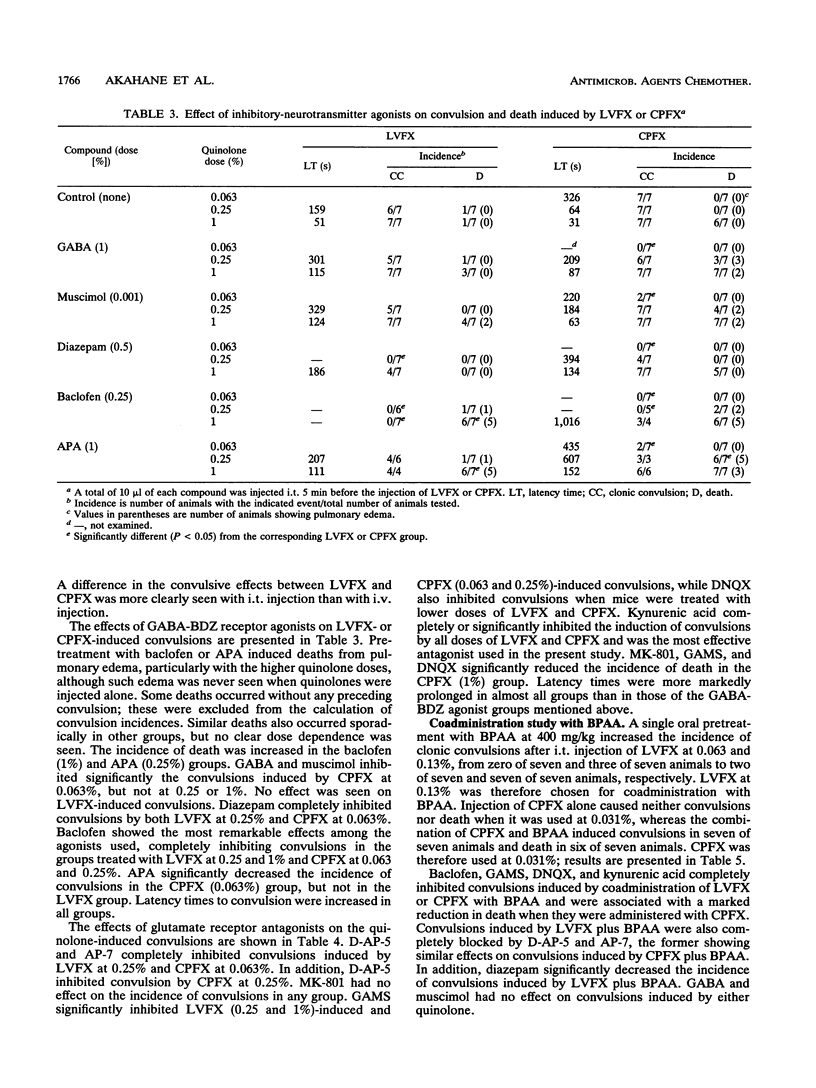
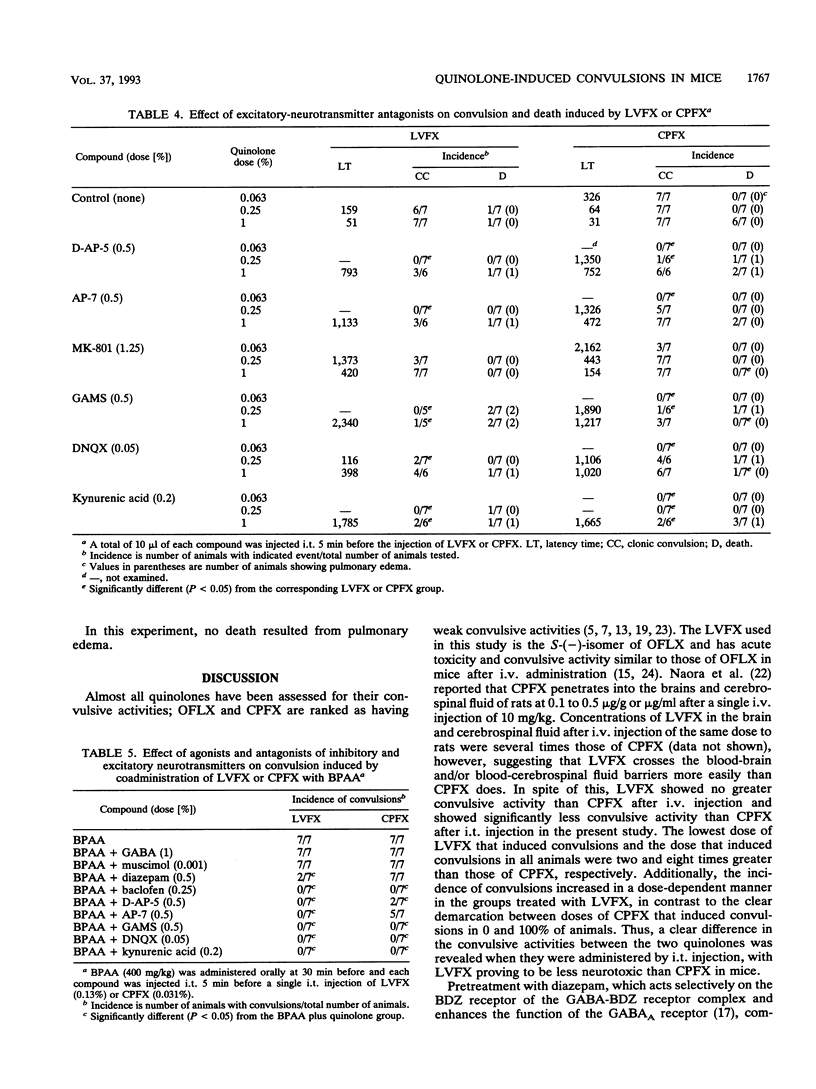
We studied the effects of gamma-aminobutyric acid (GABA)-benzodiazepine receptor agonists and glutamate receptor antagonists on levofloxacin (LVFX)- and ciprofloxacin (CPFX)-induced convulsions using intrathecal (i.t.) injections in mice. We also studied the effects of these agonists and antagonists on exacerbated convulsions induced by coadministration of the quinolone with 4-biphenylacetic acid (BPAA). The agonists or antagonists were injected i.t. 5 min and BPAA was administered orally 30 min before a single i.t. injection of the quinolone (10 microliters per animal). The animals were observed for clonic convulsion and death, and latency times to the appearance of convulsion were determined. Among the agonists, baclofen showed marked inhibition of both LVFX- and CPFX-induced convulsions, while other compounds such as GABA, muscimol, diazepam, and 3-aminopropylphosphonic acid had slight effects. Among the antagonists, kynurenic acid showed the strongest inhibition of convulsions caused by all doses of LVFX and CPFX and prolonged latency times; gamma-glutamyl-aminomethylsulfonic acid (GAMS) also markedly inhibited convulsions. The antagonists D-AP-5, AP-7, and 6,7-dinitroquinoxaline-2,3-dione (DNQX) had slight effects. Additionally, GAMS, DNQX, and MK-801 significantly lowered the incidence of death in the groups treated with CPFX. The enhanced convulsive activities of LVFX or CPFX by pretreatment with BPAA were clearly blocked by baclofen, kynurenic acid, GAMS, and DNQX. D-AP-5 and AP-7 also showed clear effects on the activity of LVFX. These results suggest that LVFX has fewer effects on the brains than CPFX and that convulsions induced by these quinolones alone and by these quinolones administered with BPAA may be mediated largely through glutamate and GABA(B) rather than GABA(A) receptors in mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akahane K., Sekiguchi M., Une T., Osada Y. Structure-epileptogenicity relationship of quinolones with special reference to their interaction with gamma-aminobutyric acid receptor sites. Antimicrob Agents Chemother. 1989 Oct;33(10):1704–1708. doi: 10.1128/aac.33.10.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio G. D., Menscer D., Little J. M., Jr Norfloxacin and seizures. Ann Intern Med. 1988 Jul 15;109(2):169–170. doi: 10.7326/0003-4819-109-2-169. [DOI] [PubMed] [Google Scholar]
- Arcieri G., Griffith E., Gruenwaldt G., Heyd A., O'Brien B., Becker N., August R. Ciprofloxacin: an update on clinical experience. Am J Med. 1987 Apr 27;82(4A):381–386. [PubMed] [Google Scholar]
- Bowery N. G., Pratt G. D. GABAB receptors as targets for drug action. Arzneimittelforschung. 1992 Feb;42(2A):215–223. [PubMed] [Google Scholar]
- Dimpfel W., Spüler M., Dalhoff A., Hofmann W., Schlüter G. Hippocampal activity in the presence of quinolones and fenbufen in vitro. Antimicrob Agents Chemother. 1991 Jun;35(6):1142–1146. doi: 10.1128/aac.35.6.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd P. R., Davies L. P., Watson W. E., Nielsen B., Dyer J. A., Wong L. S., Johnston G. A. Neurochemical studies on quinolone antibiotics: effects on glutamate, GABA and adenosine systems in mammalian CNS. Pharmacol Toxicol. 1989 May;64(5):404–411. doi: 10.1111/j.1600-0773.1989.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Enginar N., Eroğlu L. The effect of ofloxacin and ciprofloxacin on pentylenetetrazol-induced convulsions in mice. Pharmacol Biochem Behav. 1991 Jul;39(3):587–589. doi: 10.1016/0091-3057(91)90132-l. [DOI] [PubMed] [Google Scholar]
- Halliwell R. F., Davey P. G., Lambert J. J. The effects of quinolones and NSAIDs upon GABA-evoked currents recorded from rat dorsal root ganglion neurones. J Antimicrob Chemother. 1991 Feb;27(2):209–218. doi: 10.1093/jac/27.2.209. [DOI] [PubMed] [Google Scholar]
- Hara Y., Ally A., Suzuki T., Murayama S. [Effects of drugs on the convulsions induced by the combination of a new quinolone antimicrobial, enoxacin, and a nonsteroidal anti-inflammatory drug, fenbufen, in mice]. Nihon Yakurigaku Zasshi. 1992 Oct;100(4):301–305. doi: 10.1254/fpj.100.301. [DOI] [PubMed] [Google Scholar]
- Herrling P. L. Synaptic physiology of excitatory amino acids. Arzneimittelforschung. 1992 Feb;42(2A):202–208. [PubMed] [Google Scholar]
- Hirai S., Tanaka K., Makino S., Narita H. [Adverse drug interactions between pyridonecarboxylic acids and nonsteroidal antiinflammatory drugs: convulsion after oral or intracerebral administration in mice]. Yakugaku Zasshi. 1989 Feb;109(2):119–126. doi: 10.1248/yakushi1947.109.2_119. [DOI] [PubMed] [Google Scholar]
- Kato M., Furuhama K., Yoshida M., Akahane K., Takayama S. Acute oral toxicity of the new quinolone antibacterial agent levofloxacin in mice, rats and monkeys. Arzneimittelforschung. 1992 Mar;43(3A):365–366. [PubMed] [Google Scholar]
- Motomura M., Kataoka Y., Takeo G., Shibayama K., Ohishi K., Nakamura T., Niwa M., Tsujihata M., Nagataki S. Hippocampus and frontal cortex are the potential mediatory sites for convulsions induced by new quinolones and non-steroidal anti-inflammatory drugs. Int J Clin Pharmacol Ther Toxicol. 1991 Jun;29(6):223–227. [PubMed] [Google Scholar]
- Murayama S., Hara Y., Ally A., Suzuki T., Tamagawa M. [Central stimulating effect of the combination of the new quinolone group of antimicrobials and nonsteroidal anti-inflammatory drugs in mice]. Nihon Yakurigaku Zasshi. 1992 Jan;99(1):13–18. doi: 10.1254/fpj.99.13. [DOI] [PubMed] [Google Scholar]
- Möhler H. GABAergic synaptic transmission. Regulation by drugs. Arzneimittelforschung. 1992 Feb;42(2A):211–214. [PubMed] [Google Scholar]
- Naora K., Katagiri Y., Ichikawa N., Hayashibara M., Iwamoto K. A possible reduction in the renal clearance of ciprofloxacin by fenbufen in rats. J Pharm Pharmacol. 1990 Oct;42(10):704–707. doi: 10.1111/j.2042-7158.1990.tb06563.x. [DOI] [PubMed] [Google Scholar]
- Naora K., Katagiri Y., Ichikawa N., Hayashibara M., Iwamoto K. Enhanced entry of ciprofloxacin into the rat central nervous system induced by fenbufen. J Pharmacol Exp Ther. 1991 Sep;258(3):1033–1037. [PubMed] [Google Scholar]
- Rasmussen K., Krystal J. H., Aghajanian G. K. Excitatory amino acids and morphine withdrawal: differential effects of central and peripheral kynurenic acid administration. Psychopharmacology (Berl) 1991;105(4):508–512. doi: 10.1007/BF02244371. [DOI] [PubMed] [Google Scholar]
- Roussel J. P., Tapia-Arancibia L., Jourdan J., Astier H. Effect of norfloxacin, a new quinolone, on GABA modulation of TRH-induced TSH release from perifused rat pituitaries. Acta Endocrinol (Copenh) 1988 Dec;119(4):481–487. doi: 10.1530/acta.0.1190481. [DOI] [PubMed] [Google Scholar]
- Segev S., Rehavi M., Rubinstein E. Quinolones, theophylline, and diclofenac interactions with the gamma-aminobutyric acid receptor. Antimicrob Agents Chemother. 1988 Nov;32(11):1624–1626. doi: 10.1128/aac.32.11.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson K. J., Brodie M. J. Convulsions related to enoxacin. Lancet. 1985 Jul 20;2(8447):161–161. doi: 10.1016/s0140-6736(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Hara Y., Tamagawa M., Kakizaki K., Murayama S. [Effects of the combination of new quinolones and a nonsteroidal anti-inflammatory drug, fenbufen, on the EEG of rabbits]. Nihon Yakurigaku Zasshi. 1992 Jan;99(1):45–54. doi: 10.1254/fpj.99.45. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Sato H., Kume Y., Tamai I., Okezaki E., Nagata O., Kato H. Inhibitory effects of quinolone antibacterial agents on gamma-aminobutyric acid binding to receptor sites in rat brain membranes. Antimicrob Agents Chemother. 1988 Feb;32(2):190–194. doi: 10.1128/aac.32.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unseld E., Ziegler G., Gemeinhardt A., Janssen U., Klotz U. Possible interaction of fluoroquinolones with the benzodiazepine-GABAA-receptor complex. Br J Clin Pharmacol. 1990 Jul;30(1):63–70. doi: 10.1111/j.1365-2125.1990.tb03744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velísek L., Veresová S., Pôbisová H., Mares P. Excitatory amino acid antagonists and pentylenetetrazol-induced seizures during ontogenesis. II. The effects of MK-801. Psychopharmacology (Berl) 1991;104(4):510–514. doi: 10.1007/BF02245658. [DOI] [PubMed] [Google Scholar]
- Velísková J., Velísek L. S. Picrotoxin-induced tonic-clonic seizures and lethality are decreased by MK-801 in developing rats. Pharmacol Biochem Behav. 1992 Sep;43(1):291–295. doi: 10.1016/0091-3057(92)90670-b. [DOI] [PubMed] [Google Scholar]
- Velísková J., Velísek L., Mares P., Rokyta R. Ketamine suppresses both bicuculline- and picrotoxin-induced generalized tonic-clonic seizures during ontogenesis. Pharmacol Biochem Behav. 1990 Dec;37(4):667–674. doi: 10.1016/0091-3057(90)90544-r. [DOI] [PubMed] [Google Scholar]
- Williams P. D., Helton D. R. The proconvulsive activity of quinolone antibiotics in an animal model. Toxicol Lett. 1991 Sep;58(1):23–28. doi: 10.1016/0378-4274(91)90186-a. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Norfloxacin: a new targeted fluoroquinolone antimicrobial agent. Ann Intern Med. 1988 Feb;108(2):238–251. doi: 10.7326/0003-4819-108-2-238. [DOI] [PubMed] [Google Scholar]


