Abstract
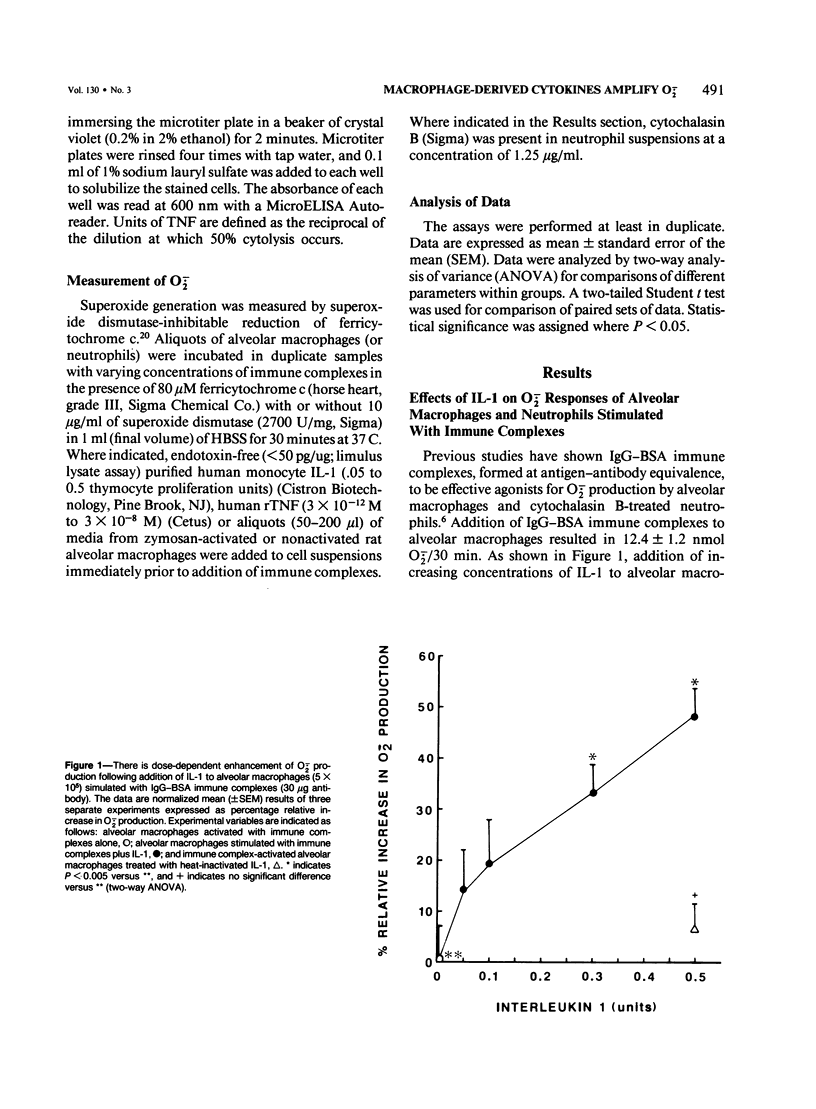
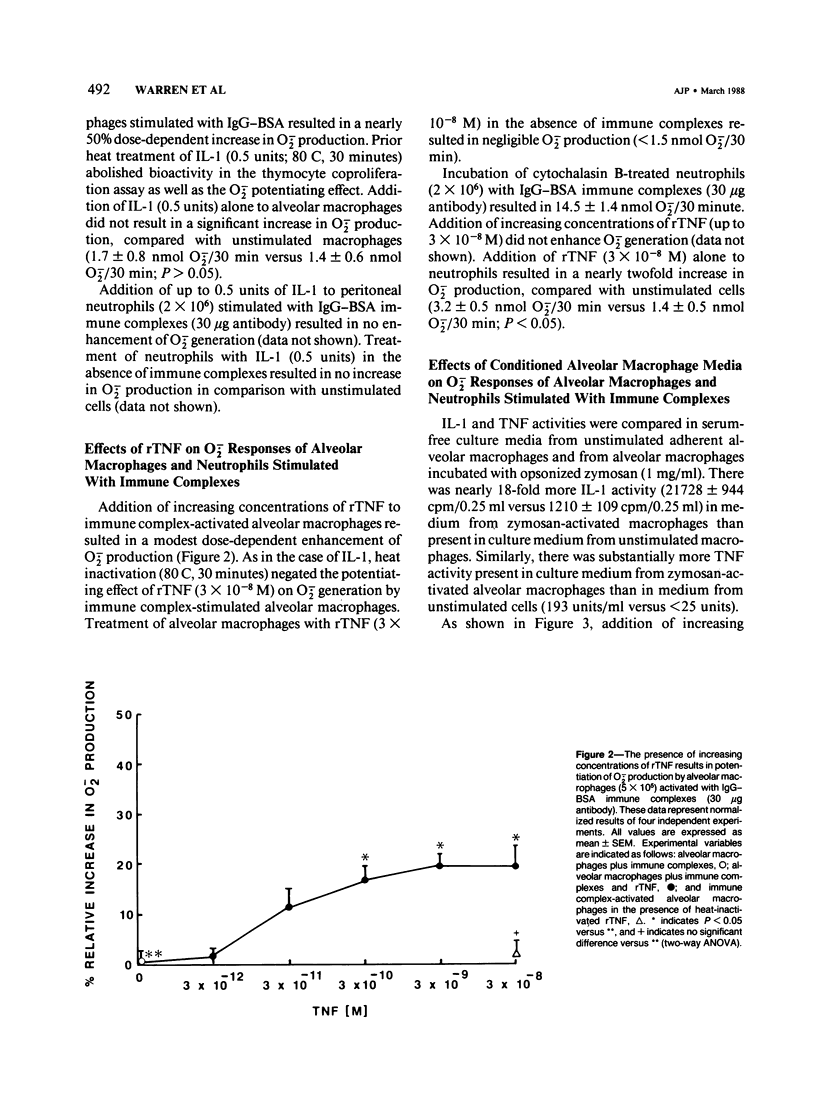
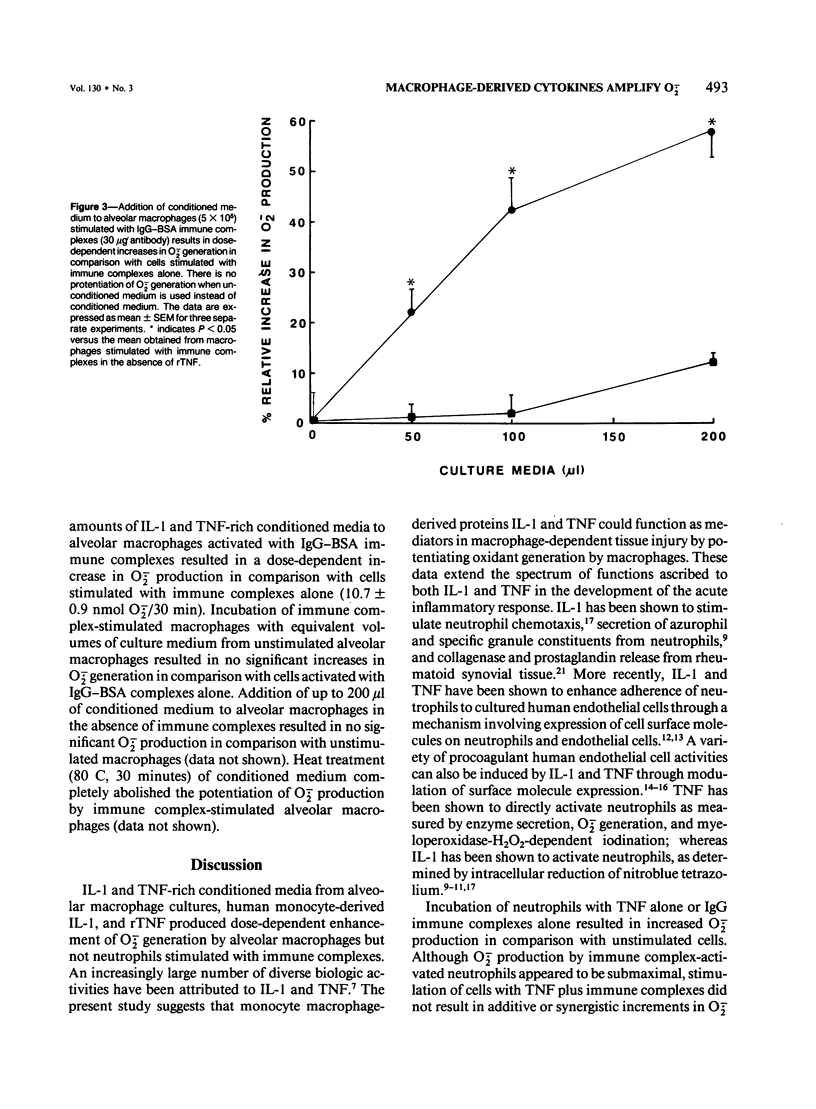
Interleukin-1 (IL-1) and tumor necrosis factor (TNF) are monocyte macrophage-derived hormonelike regulatory proteins that participate in many physiologic and pathophysiologic processes. Several proinflammatory activities have been attributed to these cytokines, but their importance in anatomically compartmentalized inflammatory processes is unclear. The current in vitro studies have been designed to examine modulatory influences of these cytokines on O2-. responses of rat phagocytes implicated as effector cells in immune complex mediated lung injury. Purified human IL-1, recombinant human TNF (rTNF), and culture supernatant from zymosan-activated alveolar macrophages significantly amplified O2-. responses of immune complex-stimulated alveolar macrophages but did not enhance the responses of neutrophils. Equivalent concentrations of IL-1, rTNF, and alveolar macrophage culture supernatant had no direct stimulatory effect on alveolar macrophages as measured by O2-. production. Culture media from unstimulated alveolar macrophages exerted negligible effects on O2-. generation by immune complex-activated alveolar macrophages. These data indicate that O2- responses of immune complex alveolar macrophages can be enhanced by the presence of IL-1, TNF, or media from activated macrophages. It is possible that macrophage products may greatly amplify tissue injury through the enhancement of oxygen radical production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Hunninghake G. W., Crystal R. G. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. J Clin Invest. 1982 Oct;70(4):806–822. doi: 10.1172/JCI110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy K. G., DeCoursey T. E., Cahalan M. D., Gupta S. Ion channels in lymphocytes. J Clin Immunol. 1985 Jan;5(1):1–6. doi: 10.1007/BF00915161. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Acute immunologic pulmonary alveolitis. J Clin Invest. 1974 Aug;54(2):349–357. doi: 10.1172/JCI107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A., Kunkel R. G., Wilson B. S. Mediation of IgA induced lung injury in the rat. Role of macrophages and reactive oxygen products. Lab Invest. 1986 May;54(5):499–506. [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- Johnson K. J., Wilson B. S., Till G. O., Ward P. A. Acute lung injury in rat caused by immunoglobulin A immune complexes. J Clin Invest. 1984 Aug;74(2):358–369. doi: 10.1172/JCI111431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Klempner M. S., Dinarello C. A., Gallin J. I. Human leukocytic pyrogen induces release of specific granule contents from human neutrophils. J Clin Invest. 1978 May;61(5):1330–1336. doi: 10.1172/JCI109050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Dayer J. M., Krane S. M., Mergenhagen S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc Natl Acad Sci U S A. 1981 Apr;78(4):2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Murphy P. A., Simon P. L., Willoughby W. F. Endogenous pyrogens made by rabbit peritoneal exudate cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol. 1980 May;124(5):2498–2501. [PubMed] [Google Scholar]
- Nedwin G. E., Svedersky L. P., Bringman T. S., Palladino M. A., Jr, Goeddel D. V. Effect of interleukin 2, interferon-gamma, and mitogens on the production of tumor necrosis factors alpha and beta. J Immunol. 1985 Oct;135(4):2492–2497. [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Saklatvala J., Sarsfield S. J., Townsend Y. Pig interleukin 1. Purification of two immunologically different leukocyte proteins that cause cartilage resorption, lymphocyte activation, and fever. J Exp Med. 1985 Oct 1;162(4):1208–1222. doi: 10.1084/jem.162.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauder D. N., Mounessa N. L., Katz S. I., Dinarello C. A., Gallin J. I. Chemotactic cytokines: the role of leukocytic pyrogen and epidermal cell thymocyte-activating factor in neutrophil chemotaxis. J Immunol. 1984 Feb;132(2):828–832. [PubMed] [Google Scholar]
- Tsujimoto M., Yokota S., Vilcek J., Weissmann G. Tumor necrosis factor provokes superoxide anion generation from neutrophils. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1094–1100. doi: 10.1016/0006-291x(86)90337-2. [DOI] [PubMed] [Google Scholar]
- Ulrich F. Studies of lymphocyte activating factor from alveolar macrophages. J Reticuloendothel Soc. 1977 Jan;21(1):33–51. [PubMed] [Google Scholar]
- Van Epps D. E., Garcia M. L. Enhancement of neutrophils function as a result of prior exposure to chemotactic factor. J Clin Invest. 1980 Aug;66(2):167–175. doi: 10.1172/JCI109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Duque R. E., Sulavik M. C., Johnson K. J. In vitro and in vivo stimulation of rat neutrophils and alveolar macrophages by immune complexes. Production of O-2 and H2O2. Am J Pathol. 1983 Mar;110(3):297–309. [PMC free article] [PubMed] [Google Scholar]
- Warren J. S., Kunkel S. L., Johnson K. J., Ward P. A. In vitro activation of rat neutrophils and alveolar macrophages with IgA and IgG immune complexes. Implications for immune complex-induced lung injury. Am J Pathol. 1987 Dec;129(3):578–588. [PMC free article] [PubMed] [Google Scholar]



