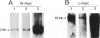Abstract
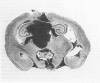
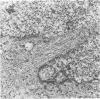
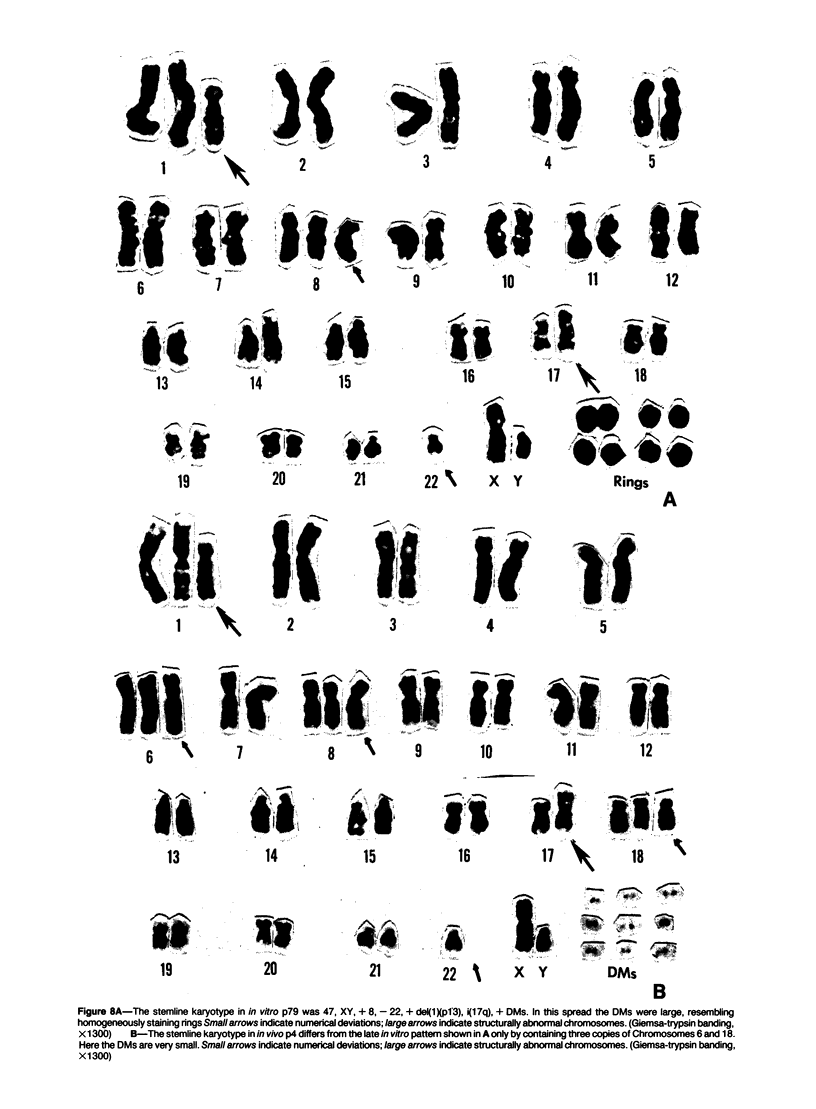
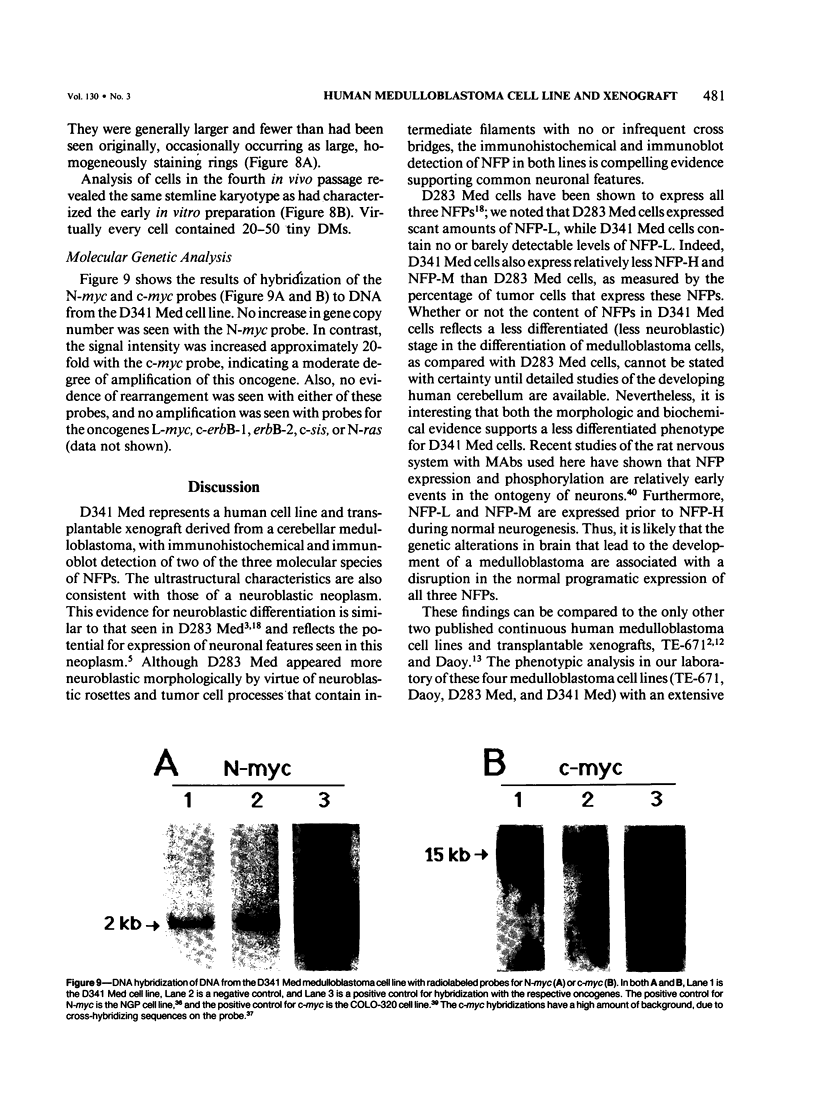
D341 Med is a new continuous cell line and transplantable xenograft derived from a cerebellar medulloblastoma. This line grew in vitro in suspension culture with spontaneous macroscopic spheroid formation and demonstrated 20-fold amplification of c-myc. Cultured D341 Med cells injected subcutaneously into athymic mice grew as markedly cellular, highly invasive undifferentiated neoplasms. Intracranial tumors grew as markedly cellular mitotically active neoplasms largely located within the subarachnoid space or lining the ventricular system. Immunocytochemical analysis of the cell line and SQ tumors revealed the high (NFP-H) and middle (NFP-M) molecular weight (Mr) neurofilament proteins (NFPs). Immunoblots demonstrated the presence of molecular species that co-migrated with authentic human NFP-H and NFP-M. This cell line and transplantable xenograft may allow, in conjunction with the authors' other models of human medulloblastoma, analysis of the heterogeneous biologic properties and therapeutic sensitivity of this tumor.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan P. M., Garson J. A., Harper E. I., Asser U., Coakham H. B., Brownell B., Kemshead J. T. Biological characterization and clinical applications of a monoclonal antibody recognizing an antigen restricted to neuroectodermal tissues. Int J Cancer. 1983 May 15;31(5):591–598. doi: 10.1002/ijc.2910310510. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):707–711. doi: 10.1073/pnas.80.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard R. O., Pambakian H. Astrocytic differentiation in medulloblastoma. J Neurol Neurosurg Psychiatry. 1980 Nov;43(11):1041–1044. doi: 10.1136/jnnp.43.11.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker L. E., Hinton D. Primitive neuroectodermal tumors of the central nervous system. Hum Pathol. 1983 Jun;14(6):538–550. doi: 10.1016/s0046-8177(83)80006-9. [DOI] [PubMed] [Google Scholar]
- Bigner D. D., Bigner S. H., Pontén J., Westermark B., Mahaley M. S., Ruoslahti E., Herschman H., Eng L. F., Wikstrand C. J. Heterogeneity of Genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981 May;40(3):201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- Bloom H. J. Medulloblastoma in children: increasing survival rates and further prospects. Int J Radiat Oncol Biol Phys. 1982 Nov;8(11):2023–2027. doi: 10.1016/0360-3016(82)90466-7. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Bullard D. E., Adams C. J., Coleman R. E., Bigner D. D. In vivo imaging of intracranial human glioma xenografts comparing specific with nonspecific radiolabeled monoclonal antibodies. J Neurosurg. 1986 Feb;64(2):257–262. doi: 10.3171/jns.1986.64.2.0257. [DOI] [PubMed] [Google Scholar]
- Bullard D. E., Bigner D. D. Heterotransplantation of human craniopharyngiomas in athymic "nude" mice. Neurosurgery. 1979 Apr;4(4):308–314. doi: 10.1227/00006123-197904000-00006. [DOI] [PubMed] [Google Scholar]
- Caputy A. J., McCullough D. C., Manz H. J., Patterson K., Hammock M. K. A review of the factors influencing the prognosis of medulloblastoma. The importance of cell differentiation. J Neurosurg. 1987 Jan;66(1):80–87. doi: 10.3171/jns.1987.66.1.0080. [DOI] [PubMed] [Google Scholar]
- Carden M. J., Trojanowski J. Q., Schlaepfer W. W., Lee V. M. Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J Neurosci. 1987 Nov;7(11):3489–3504. doi: 10.1523/JNEUROSCI.07-11-03489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coakham H. B., Garson J. A., Brownell B., Allan P. M., Harper E. I., Kemshead J. T. The use of monoclonal antibodies in the immunohistological diagnosis of cerebral and spinal tumours. S Afr J Surg. 1984 Feb-Mar;22(1):13–22. [PubMed] [Google Scholar]
- Coakham H. B., Garson J. A., Brownell B., Kemshead J. T. Diagnosis of cerebral neoplasms using monoclonal antibodies. Prog Exp Tumor Res. 1985;29:57–77. doi: 10.1159/000411626. [DOI] [PubMed] [Google Scholar]
- Coakham H. B., Garson J. A., Brownell B., Kemshead J. T. Diagnosis of cerebral neoplasms using monoclonal antibodies. Prog Exp Tumor Res. 1985;29:57–77. doi: 10.1159/000411626. [DOI] [PubMed] [Google Scholar]
- Coakham H. B., Richardson R. B., Davies A. G., Bourne S. P., Moseley R. P., Kemshead J. T., Lashford L. Antibody-guided radiation therapy via the CSF for malignant meningitis. Lancet. 1986 Oct 11;2(8511):860–861. doi: 10.1016/s0140-6736(86)92892-8. [DOI] [PubMed] [Google Scholar]
- Dranoff G., Elion G. B., Friedman H. S., Campbell G. L., Bigner D. D. Influence of glutamine on the growth of human glioma and medulloblastoma in culture. Cancer Res. 1985 Sep;45(9):4077–4081. [PubMed] [Google Scholar]
- Filmus J., Pollak M. N., Cairncross J. G., Buick R. N. Amplified, overexpressed and rearranged epidermal growth factor receptor gene in a human astrocytoma cell line. Biochem Biophys Res Commun. 1985 Aug 30;131(1):207–215. doi: 10.1016/0006-291x(85)91790-5. [DOI] [PubMed] [Google Scholar]
- Friedman H. S., Bigner S. H., McComb R. D., Schold S. C., Jr, Pasternak J. F., Groothuis D. R., Bigner D. D. A model for human medulloblastoma. Growth, morphology, and chromosomal analysis in vitro and in athymic mice. J Neuropathol Exp Neurol. 1983 Sep;42(5):485–503. doi: 10.1097/00005072-198309000-00001. [DOI] [PubMed] [Google Scholar]
- Friedman H. S., Burger P. C., Bigner S. H., Trojanowski J. Q., Wikstrand C. J., Halperin E. C., Bigner D. D. Establishment and characterization of the human medulloblastoma cell line and transplantable xenograft D283 Med. J Neuropathol Exp Neurol. 1985 Nov;44(6):592–605. doi: 10.1097/00005072-198511000-00005. [DOI] [PubMed] [Google Scholar]
- Friedman H. S., Colvin O. M., Ludeman S. M., Schold S. C., Jr, Boyd V. L., Mulhbaier L. H., Bigner D. D. Experimental chemotherapy of human medulloblastoma with classical alkylators. Cancer Res. 1986 Jun;46(6):2827–2833. [PubMed] [Google Scholar]
- Friedman H. S., Mahaley M. S., Jr, Schold S. C., Jr, Vick N. A., Falletta J. M., Bullard D. E., D'Souza B. J., Khandekar J. D., Lew S., Oakes W. J. Efficacy of vincristine and cyclophosphamide in the therapy of recurrent medulloblastoma. Neurosurgery. 1986 Mar;18(3):335–340. doi: 10.1227/00006123-198603000-00014. [DOI] [PubMed] [Google Scholar]
- Garson J. A., McIntyre P. G., Kemshead J. T. N-myc amplification in malignant astrocytoma. Lancet. 1985 Sep 28;2(8457):718–719. doi: 10.1016/s0140-6736(85)92950-2. [DOI] [PubMed] [Google Scholar]
- Jacobsen P. F., Jenkyn D. J., Papadimitriou J. M. Establishment of a human medulloblastoma cell line and its heterotransplantation into nude mice. J Neuropathol Exp Neurol. 1985 Sep;44(5):472–485. doi: 10.1097/00005072-198509000-00003. [DOI] [PubMed] [Google Scholar]
- Kapuściński J., Skoczylas B. Simple and rapid fluorimetric method for DNA microassay. Anal Biochem. 1977 Nov;83(1):252–257. doi: 10.1016/0003-2697(77)90533-4. [DOI] [PubMed] [Google Scholar]
- Kemshead J. T., Fritschy J., Garson J. A., Allan P., Coakham H., Brown S., Asser U. Monoclonal antibody UJ 127:11 detects a 220,000-240,000 kdal. glycoprotein present on a sub-set of neuroectodermally derived cells. Int J Cancer. 1983 Feb 15;31(2):187–195. doi: 10.1002/ijc.2910310209. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Bigner S. H., Bigner D. D., Trent J. M., Law M. L., O'Brien S. J., Wong A. J., Vogelstein B. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987 Apr 3;236(4797):70–73. doi: 10.1126/science.3563490. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W. Structural similarities and differences between neurofilament proteins from five different species as revealed using monoclonal antibodies. J Neurosci. 1986 Aug;6(8):2179–2186. doi: 10.1523/JNEUROSCI.06-08-02179.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W., Trojanowski J. Q. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987 Nov;7(11):3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Page C. D., Wu H. L., Schlaepfer W. W. Monoclonal antibodies to gel-excised glial filament protein and their reactivities with other intermediate filament proteins. J Neurochem. 1984 Jan;42(1):25–32. doi: 10.1111/j.1471-4159.1984.tb09692.x. [DOI] [PubMed] [Google Scholar]
- Lee V., Wu H. L., Schlaepfer W. W. Monoclonal antibodies recognize individual neurofilament triplet proteins. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6089–6092. doi: 10.1073/pnas.79.19.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libermann T. A., Nusbaum H. R., Razon N., Kris R., Lax I., Soreq H., Whittle N., Waterfield M. D., Ullrich A., Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985 Jan 10;313(5998):144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- Loeffel S. C., Gillespie G. Y., Mirmiran S. A., Miller E. W., Golden P., Askin F. B., Siegal G. P. Cellular immunolocalization of S100 protein within fixed tissue sections by monoclonal antibodies. Arch Pathol Lab Med. 1985 Feb;109(2):117–122. [PubMed] [Google Scholar]
- Marangos P. J., Zomzely-Neurath C., Luk D. C., York C. Isolation and characterization of the nervous system-specific protein 14-3-2 from rat brain. Purification, subunit composition, and comparison to the beef brain protein. J Biol Chem. 1975 Mar 10;250(5):1884–1891. [PubMed] [Google Scholar]
- McAllister R. M., Isaacs H., Rongey R., Peer M., Au W., Soukup S. W., Gardner M. B. Establishment of a human medulloblastoma cell line. Int J Cancer. 1977 Aug 15;20(2):206–212. doi: 10.1002/ijc.2910200207. [DOI] [PubMed] [Google Scholar]
- Packer R. J., Sutton L. N., Rorke L. B., Littman P. A., Sposto R., Rosenstock J. G., Bruce D. A., Schut L. Prognostic importance of cellular differentiation in medulloblastoma of childhood. J Neurosurg. 1984 Aug;61(2):296–301. doi: 10.3171/jns.1984.61.2.0296. [DOI] [PubMed] [Google Scholar]
- Pegram C. N., Eng L. F., Wikstrand C. J., McComb R. D., Lee Y. L., Bigner D. D. Monoclonal antibodies reactive with epitopes restricted to glial fibrillary acidic proteins of several species. Neurochem Pathol. 1985 Summer;3(2):119–138. doi: 10.1007/BF02834285. [DOI] [PubMed] [Google Scholar]
- Pilkington G. J., Lantos P. L. The role of glutamine synthetase in the diagnosis of cerebral tumours. Neuropathol Appl Neurobiol. 1982 May-Jun;8(3):227–236. doi: 10.1111/j.1365-2990.1982.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Rorke L. B. The cerebellar medulloblastoma and its relationship to primitive neuroectodermal tumors. J Neuropathol Exp Neurol. 1983 Jan;42(1):1–15. [PubMed] [Google Scholar]
- Rothberg P. G., Erisman M. D., Diehl R. E., Rovigatti U. G., Astrin S. M. Structure and expression of the oncogene c-myc in fresh tumor material from patients with hematopoietic malignancies. Mol Cell Biol. 1984 Jun;4(6):1096–1103. doi: 10.1128/mcb.4.6.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa H., Takeshita I., Kuramitsu M., Egami H., Fukui M., Sato Y. Neuronal and glial proteins in medulloblastomas. II. Heterotransplantation of human medulloblastoma. Anticancer Res. 1986 Sep-Oct;6(5):911–915. [PubMed] [Google Scholar]
- Schmidt M. L., Carden M. J., Lee V. M., Trojanowski J. Q. Phosphate dependent and independent neurofilament epitopes in the axonal swellings of patients with motor neuron disease and controls. Lab Invest. 1987 Mar;56(3):282–294. [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Trent J., Meltzer P., Rosenblum M., Harsh G., Kinzler K., Mashal R., Feinberg A., Vogelstein B. Evidence for rearrangement, amplification, and expression of c-myc in a human glioblastoma. Proc Natl Acad Sci U S A. 1986 Jan;83(2):470–473. doi: 10.1073/pnas.83.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski J. Q., Friedman H. S., Burger P. C., Bigner D. D. A rapidly dividing human medulloblastoma cell line (D283 MED) expresses all three neurofilament subunits. Am J Pathol. 1987 Feb;126(2):358–363. [PMC free article] [PubMed] [Google Scholar]
- Velasco M. E., Ghobrial M. W., Ross E. R. Neuron-specific enolase and neurofilament protein as markers of differentiation in medulloblastoma. Surg Neurol. 1985 Feb;23(2):177–182. doi: 10.1016/0090-3019(85)90340-4. [DOI] [PubMed] [Google Scholar]
- Vick W. W., Wikstrand C. J., Bullard D. E., Kemshead J., Coakham H. B., Schlom J., Johnston W. W., Bigner D. D., Bigner S. H. The use of a panel of monoclonal antibodies in the evaluation of cytologic specimens from the central nervous system. Acta Cytol. 1987 Nov-Dec;31(6):815–824. [PubMed] [Google Scholar]
- Vinores S. A., Rubinstein L. J. Simultaneous expression of glial fibrillary acidic (GFA) protein and neuron-specific enolase (NSE) by the same reactive or neoplastic astrocytes. Neuropathol Appl Neurobiol. 1985 Sep-Oct;11(5):349–359. doi: 10.1111/j.1365-2990.1985.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Wikstrand C. J., Bigner D. D. Expression of human fetal brain antigens by human tumors of neuroectodermal origin as defined by monoclonal antibodies. Cancer Res. 1982 Jan;42(1):267–275. [PubMed] [Google Scholar]
- Wikstrand C. J., Mahaley M. S., Bigner D. D. Surface antigenic characteristics of human glial brain tumor cells. Cancer Res. 1977 Dec;37(12):4267–4275. [PubMed] [Google Scholar]
- Wong A. J., Bigner S. H., Bigner D. D., Kinzler K. W., Hamilton S. R., Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]