Abstract
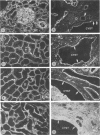
Status of basement membrane antigens in renal polycystic disease was investigated. Antibodies directed against various components of basement membrane, including anti-heparan sulfate proteoglycan, Type IV collagen, laminin, and fibronectin, were employed. Their reactivities with basement membranes of normal and cystic segments of the renal tubules were ascertained by indirect immunofluorescence. The tissues were obtained either from kidneys of patients with adult (autosomal dominant) polycystic disease or from rats with renal cystic change induced by administration of 2-amino-4,5-diphenylthiazole HCl. The human and rat tissues that had undergone cystic change exhibited essentially similar results. A loss of reactivity to anti-heparan sulfate proteoglycan antibodies was observed. The reactivity toward anti-Type IV collagen and laminin either remained unchanged or was focally increased. The reactivity toward fibronectin, normally absent, increased dramatically in the peritubular regions and interstitium. The results indicate that there is an imbalance in various antigenic components associated with renal tubular cystic disease in rat and man, which may have a fundamental role in the pathogenesis of this disorder.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black P. H. Shedding from normal and cancer-cell surfaces. N Engl J Med. 1980 Dec 11;303(24):1415–1416. doi: 10.1056/NEJM198012113032411. [DOI] [PubMed] [Google Scholar]
- Butkowski R. J., Carone F. A., Grantham J. J., Hudson B. G. Tubular basement membrane changes in 2-amino-4,5-diphenylthiazole-induced polycystic disease. Kidney Int. 1985 Nov;28(5):744–751. doi: 10.1038/ki.1985.193. [DOI] [PubMed] [Google Scholar]
- Carone F. A., Rowland R. G., Perlman S. G., Ganote C. E. The pathogenesis of drug-induced renal cystic disease. Kidney Int. 1974 Jun;5(6):411–421. doi: 10.1038/ki.1974.59. [DOI] [PubMed] [Google Scholar]
- Cuppage F. E., Huseman R. A., Chapman A., Grantham J. J. Ultrastructure and function of cysts from human adult polycystic kidneys. Kidney Int. 1980 Mar;17(3):372–381. doi: 10.1038/ki.1980.43. [DOI] [PubMed] [Google Scholar]
- Dixit S. N., Mainardi C. L., Beachey E. H., Kang A. H. 7S domain constitutes the amino-terminal end of type IV collagen: an immunochemical study. Coll Relat Res. 1983 May;3(3):263–269. doi: 10.1016/s0174-173x(83)80008-9. [DOI] [PubMed] [Google Scholar]
- Dobyan D. C., Hill D., Lewis T., Bulger R. E. Cyst formation in rat kidney induced by cis-platinum administration. Lab Invest. 1981 Sep;45(3):260–268. [PubMed] [Google Scholar]
- Evan A. P., Gardner K. D., Jr Nephron obstruction in nordihydroguaiaretic acid-induced renal cystic disease. Kidney Int. 1979 Jan;15(1):7–19. doi: 10.1038/ki.1979.2. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Donoso V. S., Evan A. P., Carone F. A., Gardner K. D., Jr Viscoelastic properties of tubule basement membranes in experimental renal cystic disease. Kidney Int. 1987 Aug;32(2):187–197. doi: 10.1038/ki.1987.191. [DOI] [PubMed] [Google Scholar]
- Grantham J. J. Polycystic kidney disease: a predominance of giant nephrons. Am J Physiol. 1983 Jan;244(1):F3–10. doi: 10.1152/ajprenal.1983.244.1.F3. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Carone F. A. Reversible changes of tubular cell and basement membrane in drug-induced renal cystic disease. Kidney Int. 1984 Jul;26(1):35–43. doi: 10.1038/ki.1984.131. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Rosenzweig L. J., Linker A., Jakubowski M. L. Decreased de novo synthesis of glomerular proteoglycans in diabetes: biochemical and autoradiographic evidence. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2272–2275. doi: 10.1073/pnas.80.8.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H., Gibbons J. T., Reddy M. K., Kanwar Y. S. Nephritogenicity of antibodies to proteoglycans of the glomerular basement membrane--I. J Clin Invest. 1986 Jan;77(1):142–156. doi: 10.1172/JCI112269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Amenta P. S. The basement membrane in pathology. Lab Invest. 1983 Jun;48(6):656–677. [PubMed] [Google Scholar]
- Rohrbach D. H., Hassell J. R., Kleinman H. K., Martin G. R. Alterations in the basement membrane (heparan sulfate) proteoglycan in diabetic mice. Diabetes. 1982 Feb;31(2):185–188. doi: 10.2337/diab.31.2.185. [DOI] [PubMed] [Google Scholar]
- Welling L. W., Grantham J. J. Physical properties of isolated perfused renal tubules and tubular basement membranes. J Clin Invest. 1972 May;51(5):1063–1075. doi: 10.1172/JCI106898. [DOI] [PMC free article] [PubMed] [Google Scholar]