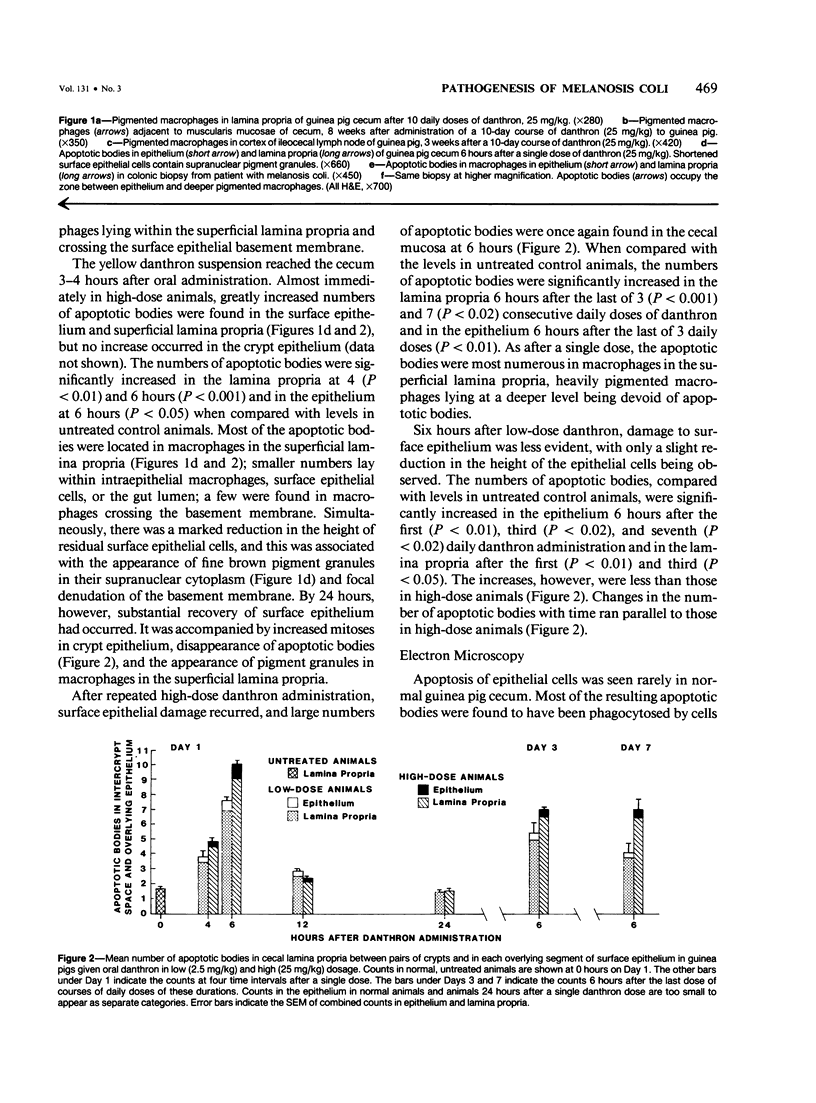
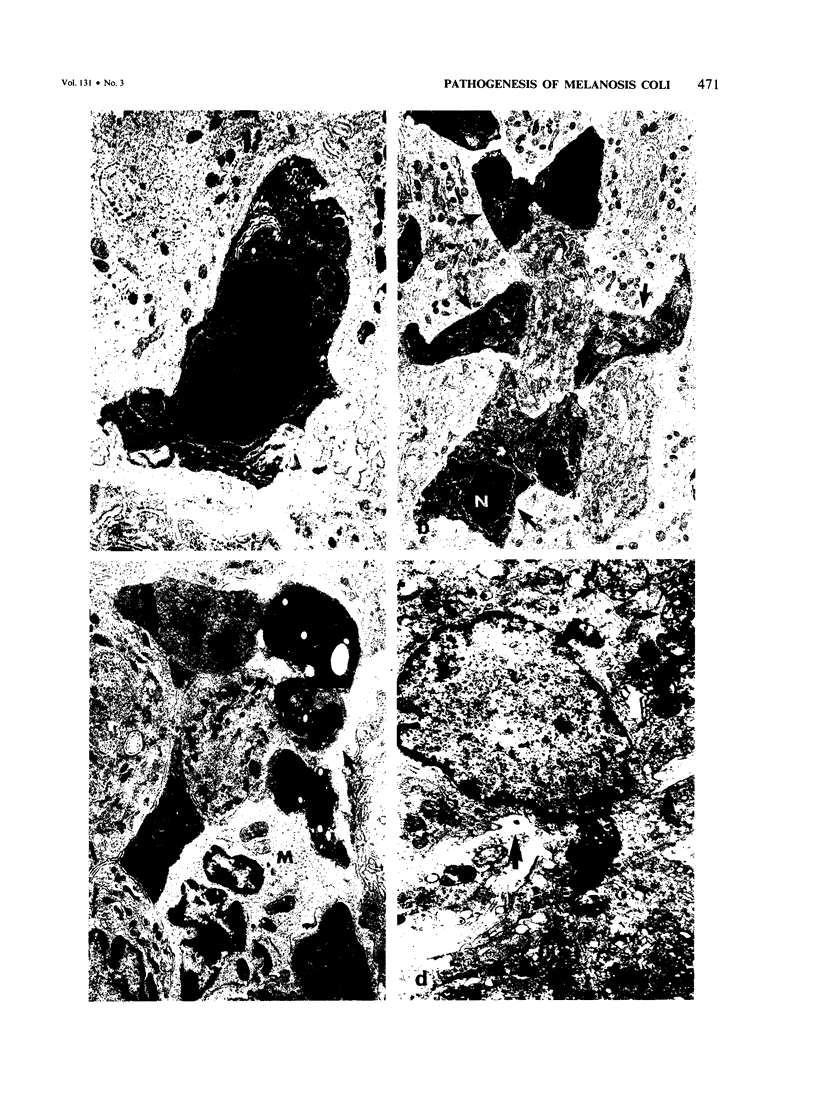
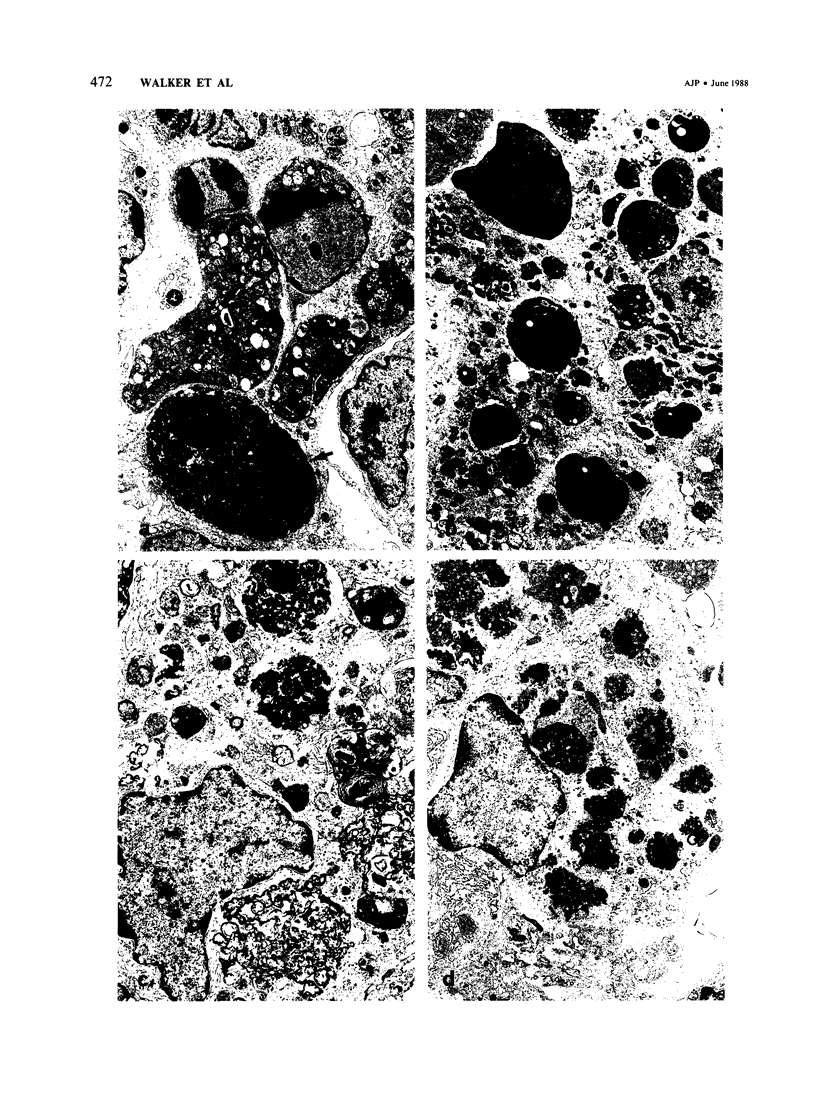
Abstract
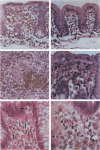
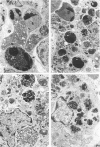
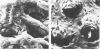
A condition closely resembling human melanosis coli was induced in the guinea pig large intestine by daily oral administration of the anthraquinone danthron. Each treatment caused a transient, dose-related wave of apoptosis of the colonic surface epithelial cells. Most of the resulting apoptotic bodies were phagocytosed by intraepithelial macrophages and carried by them through fenestrae in the epithelial basement membrane to the lamina propria. Here, the apoptotic bodies were transformed into typical lipofuscin pigment in macrophage heterolysosomes. Continued danthron administration caused progressive accumulation of pigmented macrophages in the bowel wall, whereas ongoing migration of pigmented macrophages to regional lymph nodes resulted, after danthron was ceased, in sequential loss of the pigmented cells from the superficial and deep lamina propria. Examination of colonic biopsies from patients with melanosis coli shows increased numbers of apoptotic bodies in the surface epithelium and lamina propria, suggesting implication of the same cellular processes in the formation of the pigment in man.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astaldi G., Meardi G., Lisino T. The iron content of jejunal mucosa obtained by Crosby's biopsy in hemochromatosis and hemosiderosis. Blood. 1966 Jul;28(1):70–82. [PubMed] [Google Scholar]
- Austin L. L., Dobbins W. O., 3rd Intraepithelial leukocytes of the intestinal mucosa in normal man and in Whipple's disease: a light- and electron-microscopic study. Dig Dis Sci. 1982 Apr;27(4):311–320. doi: 10.1007/BF01296750. [DOI] [PubMed] [Google Scholar]
- Badiali D., Marcheggiano A., Pallone F., Paoluzi P., Bausano G., Iannoni C., Materia E., Anzini F., Corazziari E. Melanosis of the rectum in patients with chronic constipation. Dis Colon Rectum. 1985 Apr;28(4):241–245. doi: 10.1007/BF02554044. [DOI] [PubMed] [Google Scholar]
- Brown J. P. A review of the genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutat Res. 1980 May;75(3):243–277. doi: 10.1016/0165-1110(80)90029-9. [DOI] [PubMed] [Google Scholar]
- Dillon S. B., MacDonald T. T. Functional properties of lymphocytes isolated from murine small intestinal epithelium. Immunology. 1984 Jul;52(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- Duncan A. M., Heddle J. A. The frequency and distribution of apoptosis induced by three non-carcinogenic agents in mouse colonic crypts. Cancer Lett. 1984 Jul;23(3):307–311. doi: 10.1016/0304-3835(84)90098-3. [DOI] [PubMed] [Google Scholar]
- Elmes M. E., Jones J. G. Ultrastructural studies on Paneth cell apoptosis in zinc deficient rats. Cell Tissue Res. 1980;208(1):57–63. doi: 10.1007/BF00234173. [DOI] [PubMed] [Google Scholar]
- Engster M., Abraham R. Cecal response to different molecular weights and types of carrageenan in the guinea pig. Toxicol Appl Pharmacol. 1976 Nov;38(2):265–282. doi: 10.1016/0041-008x(76)90134-4. [DOI] [PubMed] [Google Scholar]
- Epstein R. J., McDonald G. B., Sale G. E., Shulman H. M., Thomas E. D. The diagnostic accuracy of the rectal biopsy in acute graft-versus-host disease: a prospective study of thirteen patients. Gastroenterology. 1980 Apr;78(4):764–771. [PubMed] [Google Scholar]
- Gallucci B. B., Sale G. E., McDonald G. B., Epstein R., Shulman H. M., Thomas E. D. The fine structure of human rectal epithelium in acute graft-versus-host disease. Am J Surg Pathol. 1982 Jun;6(4):293–305. doi: 10.1097/00000478-198206000-00002. [DOI] [PubMed] [Google Scholar]
- Ghadially F. N. Ganglion cysts of bones. Br Med J. 1972 Feb 19;1(5798):508–509. doi: 10.1136/bmj.1.5798.508-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadially F. N., Parry E. W. An electron-microscope and histochemical study of melanosis coli. J Pathol Bacteriol. 1966 Oct;92(2):313–317. doi: 10.1002/path.1700920207. [DOI] [PubMed] [Google Scholar]
- Gobe G. C., Axelsen R. A. Genesis of renal tubular atrophy in experimental hydronephrosis in the rat. Role of apoptosis. Lab Invest. 1987 Mar;56(3):273–281. [PubMed] [Google Scholar]
- Griffiths G. D., Leek M. D., Gee D. J. The toxic plant proteins ricin and abrin induce apoptotic changes in mammalian lymphoid tissues and intestine. J Pathol. 1987 Mar;151(3):221–229. doi: 10.1002/path.1711510310. [DOI] [PubMed] [Google Scholar]
- Ijiri K., Potten C. S. Further studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agents. Br J Cancer. 1987 Feb;55(2):113–123. doi: 10.1038/bjc.1987.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijiri K., Potten C. S. Response of intestinal cells of differing topographical and hierarchical status to ten cytotoxic drugs and five sources of radiation. Br J Cancer. 1983 Feb;47(2):175–185. doi: 10.1038/bjc.1983.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D. D., Laissue J. A., LeFevre M. E. Distribution and fate of ingested carbon particles in mice. J Reticuloendothel Soc. 1978 Nov;24(5):477–487. [PubMed] [Google Scholar]
- Keenan K. P., Sharpnack D. D., Collins H., Formal S. B., O'Brien A. D. Morphologic evaluation of the effects of Shiga toxin and E coli Shiga-like toxin on the rabbit intestine. Am J Pathol. 1986 Oct;125(1):69–80. [PMC free article] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro T. Fenestrations of the basal lamina of intestinal villi of the rat. Scanning and transmission electron microscopy. Cell Tissue Res. 1985;239(1):183–188. doi: 10.1007/BF00214918. [DOI] [PubMed] [Google Scholar]
- Kotler D. P., Gaetz H. P., Lange M., Klein E. B., Holt P. R. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1984 Oct;101(4):421–428. doi: 10.7326/0003-4819-101-4-421. [DOI] [PubMed] [Google Scholar]
- Kotler D. P., Weaver S. C., Terzakis J. A. Ultrastructural features of epithelial cell degeneration in rectal crypts of patients with AIDS. Am J Surg Pathol. 1986 Aug;10(8):531–538. doi: 10.1097/00000478-198608000-00002. [DOI] [PubMed] [Google Scholar]
- LeFevre M. E., Olivo R., Vanderhoff J. W., Joel D. D. Accumulation of latex in Peyer's patches and its subsequent appearance in villi and mesenteric lymph nodes. Proc Soc Exp Biol Med. 1978 Nov;159(2):298–302. doi: 10.3181/00379727-159-40336. [DOI] [PubMed] [Google Scholar]
- Low F. N., McClugage S. G. Microdissection by ultrasonication: scanning electron microscopy of the epithelial basal lamina of the alimentary canal in the rat. Am J Anat. 1984 Feb;169(2):137–147. doi: 10.1002/aja.1001690203. [DOI] [PubMed] [Google Scholar]
- McClugage S. G., Low F. N. Microdissection by ultrasonication: porosity of the intestinal epithelial basal lamina. Am J Anat. 1984 Oct;171(2):207–216. doi: 10.1002/aja.1001710206. [DOI] [PubMed] [Google Scholar]
- Mori H., Kawai K., Ohbayashi F., Kuniyasu T., Yamazaki M., Hamasaki T., Williams G. M. Genotoxicity of a variety of mycotoxins in the hepatocyte primary culture/DNA repair test using rat and mouse hepatocytes. Cancer Res. 1984 Jul;44(7):2918–2923. [PubMed] [Google Scholar]
- Mori H., Sugie S., Niwa K., Takahashi M., Kawai K. Induction of intestinal tumours in rats by chrysazin. Br J Cancer. 1985 Nov;52(5):781–783. doi: 10.1038/bjc.1985.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Sugie S., Niwa K., Yoshimi N., Tanaka T., Hirono I. Carcinogenicity of chrysazin in large intestine and liver of mice. Jpn J Cancer Res. 1986 Sep;77(9):871–876. [PubMed] [Google Scholar]
- Owen R. L., Allen C. L., Stevens D. P. Phagocytosis of Giardia muris by macrophages in Peyer's patch epithelium in mice. Infect Immun. 1981 Aug;33(2):591–601. doi: 10.1128/iai.33.2.591-601.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977 Oct 6;269(5628):518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- Ronen A., Heddle J. A. Site-specific induction of nuclear anomalies (apoptotic bodies and micronuclei) by carcinogens in mice. Cancer Res. 1984 Apr;44(4):1536–1540. [PubMed] [Google Scholar]
- Russell N. J., Royland J. E., McCawley E. L. Danthron induced melanosis coli in the guinea pig. Proc West Pharmacol Soc. 1980;23:277–280. [PubMed] [Google Scholar]
- Russell N. J., Royland J. E., McCawley E. L. Melanosis coli in the guinea pig: a light and electron microscopic study. Proc West Pharmacol Soc. 1982;25:193–195. [PubMed] [Google Scholar]
- SCHRODT G. R. MELANOSIS COLI: A STUDY WITH THE ELECTRON MICROSCOPE. Dis Colon Rectum. 1963 Jul-Aug;6:277–283. doi: 10.1007/BF02617266. [DOI] [PubMed] [Google Scholar]
- SPEARE G. S. Melanosis coll; experimental observations on its production and elimination in twenty-three cases. Am J Surg. 1951 Nov;82(5):631–637. doi: 10.1016/0002-9610(51)90432-1. [DOI] [PubMed] [Google Scholar]
- Sawicki W., Kucharczyk K., Szymanska K., Kujawa M. Lamina propria macrophages of intestine of the guinea pig. Possible role in phagocytosis of migrating cells. Gastroenterology. 1977 Dec;73(6):1340–1344. [PubMed] [Google Scholar]
- Steer H. W., Colin-Jones D. G. Melanosis coli: studies of the toxic effects of irritant purgatives. J Pathol. 1975 Apr;115(4):199–205. doi: 10.1002/path.1711150403. [DOI] [PubMed] [Google Scholar]
- Swanbeck G., Zetterberg G. Studies on dithranol and dithranol-like compounds. I. Binding to nucleic acids. Acta Derm Venereol. 1971;51(1):41–44. [PubMed] [Google Scholar]
- Takeuchi A., Jervis H. R., Sprinz H. The globule leucocyte in the intestinal mucosa of the cat: a histochemical, light and electron microscopic study. Anat Rec. 1969 May;164(1):79–99. doi: 10.1002/ar.1091640106. [DOI] [PubMed] [Google Scholar]
- Tikkanen L., Matsushima T., Natori S. Mutagenicity of anthraquinones in the Salmonella preincubation test. Mutat Res. 1983 Mar;116(3-4):297–304. doi: 10.1016/0165-1218(83)90067-8. [DOI] [PubMed] [Google Scholar]
- WITTOESCH J. H., JACKMAN R. J., McDONALD J. R. Melanosis coli: general review and a study of 887 cases. Dis Colon Rectum. 1958 May-Jun;1(3):172–180. doi: 10.1007/BF02616828. [DOI] [PubMed] [Google Scholar]
- Walker N. I., Gobé G. C. Cell death and cell proliferation during atrophy of the rat parotid gland induced by duct obstruction. J Pathol. 1987 Dec;153(4):333–344. doi: 10.1002/path.1711530407. [DOI] [PubMed] [Google Scholar]
- Walker N. I. Ultrastructure of the rat pancreas after experimental duct ligation. I. The role of apoptosis and intraepithelial macrophages in acinar cell deletion. Am J Pathol. 1987 Mar;126(3):439–451. [PMC free article] [PubMed] [Google Scholar]
- Warfel K. A., Hull M. T. Migration of lymphocytes through the cutaneous basal lamina in normal skin: an ultrastructural study. Anat Rec. 1984 Mar;208(3):349–355. doi: 10.1002/ar.1092080305. [DOI] [PubMed] [Google Scholar]
- Wargovich M. J., Goldberg M. T., Newmark H. L., Bruce W. R. Nuclear aberrations as a short-term test for genotoxicity to the colon: evaluation of nineteen agents in mice. J Natl Cancer Inst. 1983 Jul;71(1):133–137. [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Zetterberg G., Swanbeck G. Studies on dithranol and dithranol-like compounds. II. Mutagenicity. Acta Derm Venereol. 1971;51(1):45–49. [PubMed] [Google Scholar]