Abstract
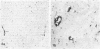
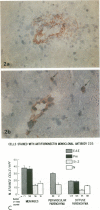
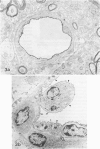
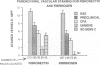
Cellular expression and extracellular deposition of fibronectin and fibrin/fibrinogen in experimental allergic encephalomyelitis (EAE) in guinea pigs (GP) were studied by light and electron microscopic immunohistochemistry of the central nervous system (CNS). Normal GPs had few fibronectin+ and fibrin/fibrinogen+ CNS vessels. Positively stained vessels were more numberous in sensitized GPs prior to and after onset of EAE, and there were extracellular deposits of these molecules in inflammatory foci. Staining was greater in EAE-susceptible strain 13 GPs than in sensitized EAE-resistant strain 2 GP. On ultrastructural analysis both molecules were localized on endothelial cell luminal surfaces and on intravascular mononuclear cells. Fibronectin and the macrophage fibronectin receptor (A6F10) were found on mononuclear cells in parenchyma. Endothelial cell luminal surface fibronectin and fibrin/fibrinogen may mark and/or contribute to early immune events in EAE, including mononuclear cell margination and emigration in inflammatory sites. Genetic factors, cytokines, and components of coagulation may regulate these interactions in vivo.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing D. H., Almeda S., Isliker H., Lahav J., Hynes R. O. Fibronectin binds to the C1q component of complement. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4198–4201. doi: 10.1073/pnas.79.13.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. J., Juliano R. L. Selective inhibition of fibronectin-mediated cell adhesion by monoclonal antibodies to a cell-surface glycoprotein. Science. 1985 Jun 21;228(4706):1448–1451. doi: 10.1126/science.4012302. [DOI] [PubMed] [Google Scholar]
- Cammer W., Brosnan C. F., Basile C., Bloom B. R., Norton W. T. Complement potentiates the degradation of myelin proteins by plasmin: implications for a mechanism of inflammatory demyelination. Brain Res. 1986 Jan 29;364(1):91–101. doi: 10.1016/0006-8993(86)90990-x. [DOI] [PubMed] [Google Scholar]
- Cavender D., Saegusa Y., Ziff M. Stimulation of endothelial cell binding of lymphocytes by tumor necrosis factor. J Immunol. 1987 Sep 15;139(6):1855–1860. [PubMed] [Google Scholar]
- Charo I. F., Fitzgerald L. A., Steiner B., Rall S. C., Jr, Bekeart L. S., Phillips D. R. Platelet glycoproteins IIb and IIIa: evidence for a family of immunologically and structurally related glycoproteins in mammalian cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8351–8355. doi: 10.1073/pnas.83.21.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciano P. S., Colvin R. B., Dvorak A. M., McDonagh J., Dvorak H. F. Macrophage migration in fibrin gel matrices. Lab Invest. 1986 Jan;54(1):62–70. [PubMed] [Google Scholar]
- Clark R. A., Dvorak H. F., Colvin R. B. Fibronectin in delayed-type hypersensitivity skin reactions: associations with vessel permeability and endothelial cell activation. J Immunol. 1981 Feb;126(2):787–793. [PubMed] [Google Scholar]
- Clark R. A., Quinn J. H., Winn H. J., Lanigan J. M., Dellepella P., Colvin R. B. Fibronectin is produced by blood vessels in response to injury. J Exp Med. 1982 Aug 1;156(2):646–651. doi: 10.1084/jem.156.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofano F., Comoglio P. M., Landolfo S., Tarone G. Mouse immune interferon enhances fibronectin production of elicited macrophages. J Immunol. 1984 Dec;133(6):3102–3106. [PubMed] [Google Scholar]
- Collins T., Lapierre L. A., Fiers W., Strominger J. L., Pober J. S. Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A,B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1986 Jan;83(2):446–450. doi: 10.1073/pnas.83.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin R. B. Fibrinogen-fibrin interactions with fibroblasts and macrophages. Ann N Y Acad Sci. 1983 Jun 27;408:621–633. doi: 10.1111/j.1749-6632.1983.tb23279.x. [DOI] [PubMed] [Google Scholar]
- Colvin R. B., Johnson R. A., Mihm M. C., Jr, Dvorak H. F. Role of the clotting system in cell-mediated hypersensitivity. I. Fibrin deposition in delayed skin reactions in man. J Exp Med. 1973 Sep 1;138(3):686–698. doi: 10.1084/jem.138.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czop J. K., Austen K. F. Augmentation of phagocytosis by a specific fibronectin fragment that links particulate activators to the fibronectin adherence receptor of human monocytes. J Immunol. 1982 Dec;129(6):2678–2681. [PubMed] [Google Scholar]
- Dejana E., Colella S., Languino L. R., Balconi G., Corbascio G. C., Marchisio P. C. Fibrinogen induces adhesion, spreading, and microfilament organization of human endothelial cells in vitro. J Cell Biol. 1987 May;104(5):1403–1411. doi: 10.1083/jcb.104.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Senger D. R., Dvorak A. M., Harvey V. S., McDonagh J. Regulation of extravascular coagulation by microvascular permeability. Science. 1985 Mar 1;227(4690):1059–1061. doi: 10.1126/science.3975602. [DOI] [PubMed] [Google Scholar]
- Edgington T. S., Curtiss L. K., Plow E. F. A linkage between the hemostatic and immune systems embodied in the fibrinolytic release of lymphocyte suppressive peptides. J Immunol. 1985 Jan;134(1):471–477. [PubMed] [Google Scholar]
- Fujikawa L. S., Chan C. C., McAllister C., Gery I., Hooks J. J., Detrick B., Nussenblatt R. B. Retinal vascular endothelium expresses fibronectin and class II histocompatibility complex antigens in experimental autoimmune uveitis. Cell Immunol. 1987 Apr 15;106(1):139–150. doi: 10.1016/0008-8749(87)90157-2. [DOI] [PubMed] [Google Scholar]
- Gardner J. M., Hynes R. O. Interaction of fibronectin with its receptor on platelets. Cell. 1985 Sep;42(2):439–448. doi: 10.1016/0092-8674(85)90101-1. [DOI] [PubMed] [Google Scholar]
- Geczy C. L., Roberts I. M., Meyer P., Bernard C. C. Susceptibility and resistance to experimental autoimmune encephalomyelitis and neuritis in the guinea pig correlate with the induction of procoagulant and anticoagulant activities. J Immunol. 1984 Dec;133(6):3026–3036. [PubMed] [Google Scholar]
- Gilboa N., Kaplan J. E. Plasma fibronectin enhances fibrinolytic system in vitro. Thromb Haemost. 1985 Oct 30;54(3):639–644. [PubMed] [Google Scholar]
- Godfrey H. P., Angadi C. V., Wolstencroft R. A., Bianco C. Localization of macrophage agglutination factor activity to the gelatin-binding domain of fibronectin. J Immunol. 1984 Sep;133(3):1417–1423. [PubMed] [Google Scholar]
- Hill H. R., Shigeoka A. O., Augustine N. H., Pritchard D., Lundblad J. L., Schwartz R. S. Fibronectin enhances the opsonic and protective activity of monoclonal and polyclonal antibody against group B streptococci. J Exp Med. 1984 Jun 1;159(6):1618–1628. doi: 10.1084/jem.159.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosein B., Bianco C. Monocyte receptors for fibronectin characterized by a monoclonal antibody that interferes with receptor activity. J Exp Med. 1985 Jul 1;162(1):157–170. doi: 10.1084/jem.162.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhler M., Barry D. I., Offner H., Konat G., Klinken L., Paulson O. B. Blood-brain and blood-spinal cord barrier permeability during the course of experimental allergic encephalomyelitis in the rat. Brain Res. 1984 Jun 8;302(2):347–355. doi: 10.1016/0006-8993(84)90249-x. [DOI] [PubMed] [Google Scholar]
- Koh C. S., Paterson P. Y. Suppression of clinical signs of cell-transferred experimental allergic encephalomyelitis and altered cerebrovascular permeability in Lewis rats treated with a plasminogen activator inhibitor. Cell Immunol. 1987 Jun;107(1):52–63. doi: 10.1016/0008-8749(87)90265-6. [DOI] [PubMed] [Google Scholar]
- Kradin R. L., Zhu Y., Hales C. A., Bianco C., Colvin R. B. Response of pulmonary macrophages to hyperoxic pulmonary injury. Acquisition of surface fibronectin and fibrin/ogen and enhanced expression of a fibronectin receptor. Am J Pathol. 1986 Nov;125(2):349–357. [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn D. M., Bargatze R. F., Butcher E. C. Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol. 1987 Jun 15;138(12):4313–4321. [PubMed] [Google Scholar]
- Liu W. T., Vanguri P., Shin M. L. Studies on demyelination in vitro: the requirement of membrane attack components of the complement system. J Immunol. 1983 Aug;131(2):778–782. [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Majeau G. R., Unanue E. R., Cotran R. S. Stimulation of human monocyte/macrophage-derived growth factor (MDGF) production by plasma fibronectin. Am J Pathol. 1983 Jun;111(3):367–373. [PMC free article] [PubMed] [Google Scholar]
- Masuyama J., Minato N., Kano S. Mechanisms of lymphocyte adhesion to human vascular endothelial cells in culture. T lymphocyte adhesion to endothelial cells through endothelial HLA-DR antigens induced by gamma interferon. J Clin Invest. 1986 May;77(5):1596–1605. doi: 10.1172/JCI112475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron R. M., Spatz M., Kempski O., Hogan R. N., Muehl L., McFarlin D. E. Interaction between myelin basic protein-sensitized T lymphocytes and murine cerebral vascular endothelial cells. J Immunol. 1986 Dec 1;137(11):3428–3435. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Norris D. A., Clark R. A., Swigart L. M., Huff J. C., Weston W. L., Howell S. E. Fibronectin fragment(s) are chemotactic for human peripheral blood monocytes. J Immunol. 1982 Oct;129(4):1612–1618. [PubMed] [Google Scholar]
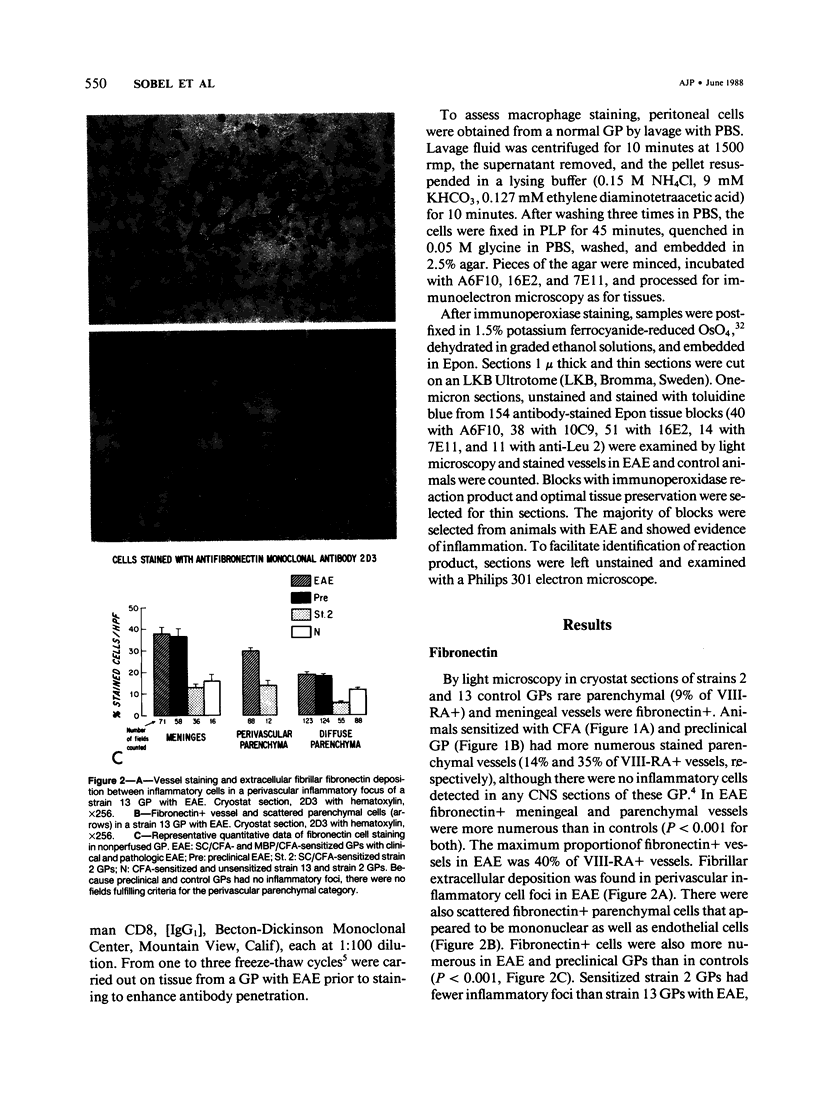
- Oldstone M. B., Dixon F. J. Immunohistochemical study of allergic encephalomyelitis. Am J Pathol. 1968 Feb;52(2):251–263. [PMC free article] [PubMed] [Google Scholar]
- Paterson P. Y., Koh C. S., Kwaan H. C. Role of the clotting system in the pathogenesis of neuroimmunologic disease. Fed Proc. 1987 Jan;46(1):91–96. [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Lapierre L. A., Stolpen A. H., Brock T. A., Springer T. A., Fiers W., Bevilacqua M. P., Mendrick D. L., Gimbrone M. A., Jr Activation of cultured human endothelial cells by recombinant lymphotoxin: comparison with tumor necrosis factor and interleukin 1 species. J Immunol. 1987 May 15;138(10):3319–3324. [PubMed] [Google Scholar]
- Raine C. S. Biology of disease. Analysis of autoimmune demyelination: its impact upon multiple sclerosis. Lab Invest. 1984 Jun;50(6):608–635. [PubMed] [Google Scholar]
- Reiber H., Suckling A. J., Rumsby M. G. The effect of Freund's adjuvants on blood-cerebrospinal fluid barrier permeability. J Neurol Sci. 1984 Jan;63(1):55–61. doi: 10.1016/0022-510x(84)90108-4. [DOI] [PubMed] [Google Scholar]
- Rowland F. N., Donovan M. J., Picciano P. T., Wilner G. D., Kreutzer D. L. Fibrin-mediated vascular injury. Identification of fibrin peptides that mediate endothelial cell retraction. Am J Pathol. 1984 Dec;117(3):418–428. [PMC free article] [PubMed] [Google Scholar]
- Skoskiewicz M. J., Colvin R. B., Schneeberger E. E., Russell P. S. Widespread and selective induction of major histocompatibility complex-determined antigens in vivo by gamma interferon. J Exp Med. 1985 Nov 1;162(5):1645–1664. doi: 10.1084/jem.162.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel R. A., Blanchette B. W., Bhan A. K., Colvin R. B. The immunopathology of experimental allergic encephalomyelitis. I. Quantitative analysis of inflammatory cells in situ. J Immunol. 1984 May;132(5):2393–2401. [PubMed] [Google Scholar]
- Sobel R. A., Blanchette B. W., Bhan A. K., Colvin R. B. The immunopathology of experimental allergic encephalomyelitis. II. Endothelial cell Ia increases prior to inflammatory cell infiltration. J Immunol. 1984 May;132(5):2402–2407. [PubMed] [Google Scholar]
- Sobel R. A., Colvin R. B. The immunopathology of experimental allergic encephalomyelitis (EAE). III. Differential in situ expression of strain 13 Ia on endothelial and inflammatory cells of (strain 2 x strain 13)F1 guinea pigs with EAE. J Immunol. 1985 Apr;134(4):2333–2337. [PubMed] [Google Scholar]
- Sobel R. A., Natale J. M., Schneeberger E. E. The immunopathology of acute experimental allergic encephalomyelitis. IV. An ultrastructural immunocytochemical study of class II major histocompatibility complex molecule (Ia) expression. J Neuropathol Exp Neurol. 1987 May;46(3):239–249. doi: 10.1097/00005072-198705000-00001. [DOI] [PubMed] [Google Scholar]
- Spies T., Morton C. C., Nedospasov S. A., Fiers W., Pious D., Strominger J. L. Genes for the tumor necrosis factors alpha and beta are linked to the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8699–8702. doi: 10.1073/pnas.83.22.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Stern D. M., Bank I., Nawroth P. P., Cassimeris J., Kisiel W., Fenton J. W., 2nd, Dinarello C., Chess L., Jaffe E. A. Self-regulation of procoagulant events on the endothelial cell surface. J Exp Med. 1985 Oct 1;162(4):1223–1235. doi: 10.1084/jem.162.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis W. J., Harlan J. M. Effector functions of endothelium in inflammatory and immunologic reactions. Pathol Immunopathol Res. 1986;5(2):73–103. doi: 10.1159/000157005. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Craigmyle L. S., Silverstein S. C. Fibronectin and serum amyloid P component stimulate C3b- and C3bi-mediated phagocytosis in cultured human monocytes. J Exp Med. 1983 Oct 1;158(4):1338–1343. doi: 10.1084/jem.158.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]