Abstract
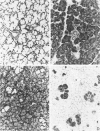
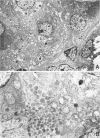
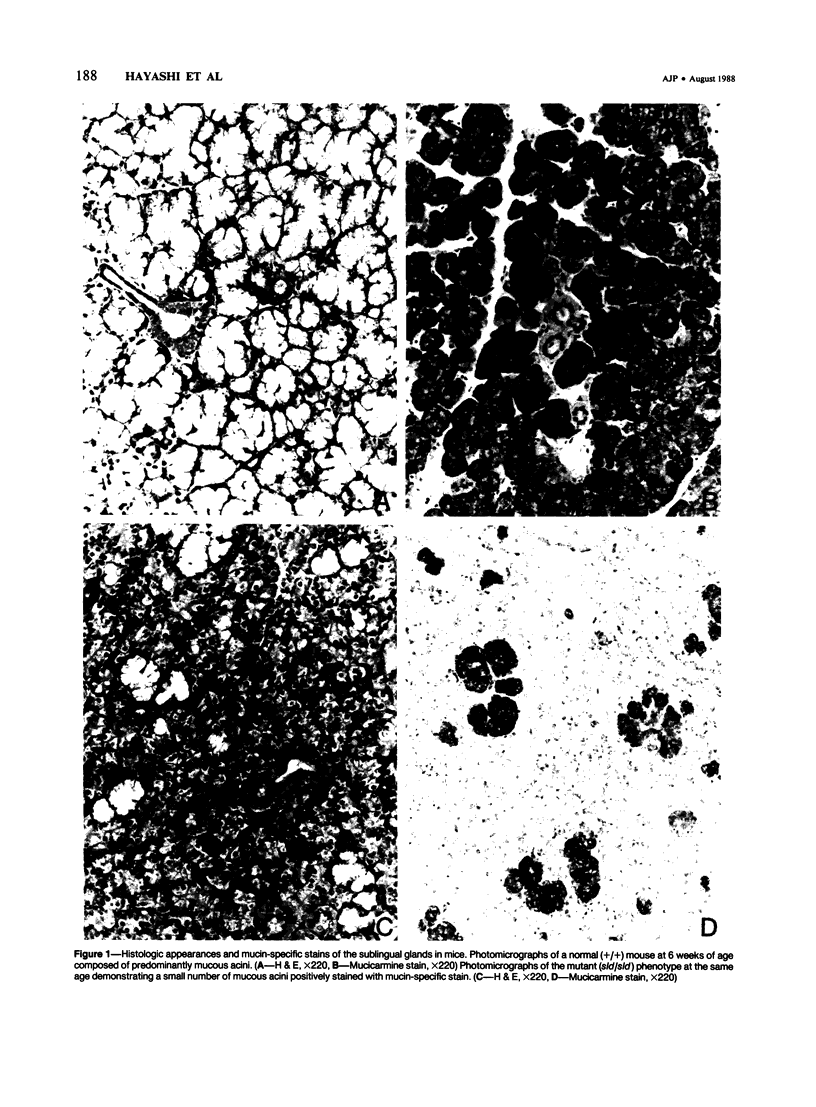
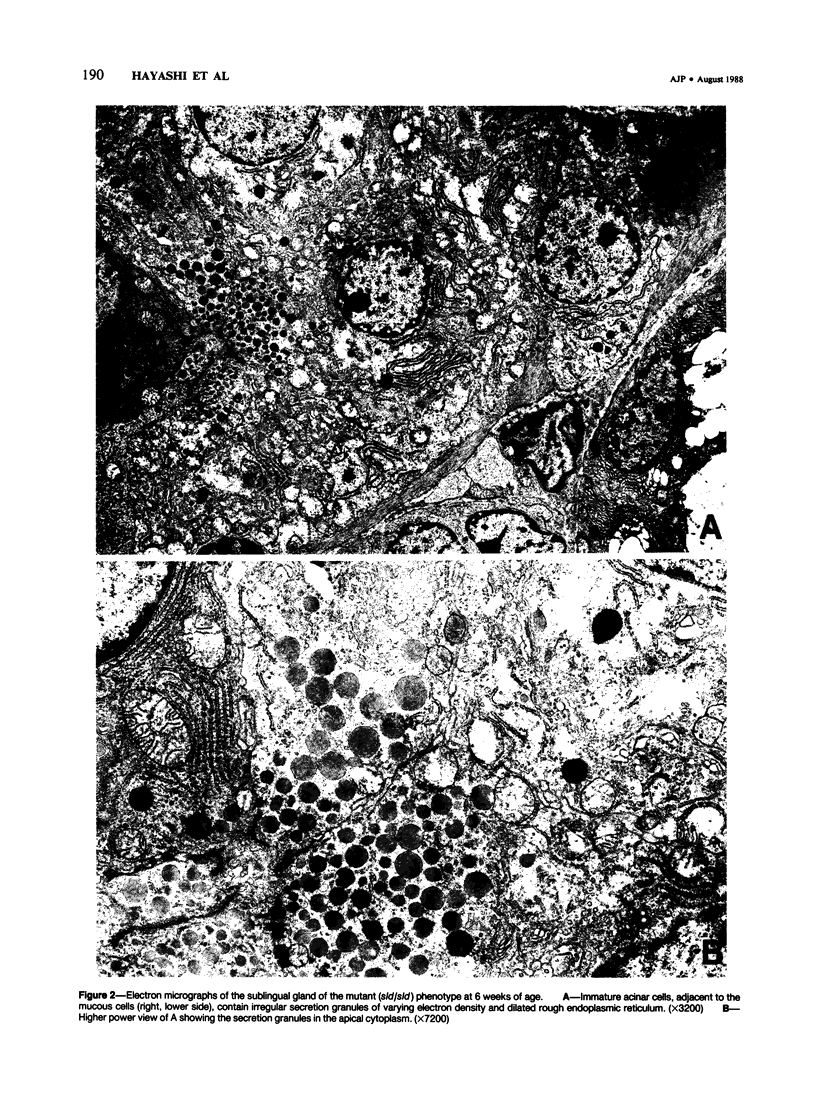
A new mutation in mice affecting the mucous cell differentiation of the sublingual glands is described. The normal mouse sublingual glands are mucus-secreting and virtually all the acinar cells differentiate to mucus-rich cells by the day of birth. In contrast, all endpieces of newborn mutant mice consisted of acini of immature cuboidal cells. However, normal mucous cells, staining intensively with mucin-specific stains such as Alcian blue at pH 2.5 or mucicarmine, appeared in the mutant mice from an early age singly or in groups in a small number of acini, and their number apparently increased with age to occupy over 30% of the total acinar cells. Ultrastructurally, irregular secretion granules of varying electron-density, distinct from ordinary sublingual mucin granules, were frequently observed in the cytoplasm of the immature acinar cells in the mutant phenotype. The genetic analysis showed that a single autosomal recessive gene determined the observed abnormality. This is the first salivary gland mutation and will provide a critical model for the study of salivary mucous cell differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caramia F. Ultrastructure of mouse submaxillary gland. I. Sexual differences. J Ultrastruct Res. 1966 Dec;16(5):505–523. doi: 10.1016/s0022-5320(66)80003-5. [DOI] [PubMed] [Google Scholar]
- Dawes C., Wood C. M. The composition of human lip mucous gland secretions. Arch Oral Biol. 1973 Mar;18(3):343–350. doi: 10.1016/0003-9969(73)90157-x. [DOI] [PubMed] [Google Scholar]
- MUNGER B. L. HISTOCHEMICAL STUDIES ON SEROMUCOUS- AND MUCOUS-SECRETING CELLS OF HUMAN SALIVARY GLANDS. Am J Anat. 1964 Nov;115:411–429. doi: 10.1002/aja.1001150303. [DOI] [PubMed] [Google Scholar]
- Redman R. S., Sweney L. R., McLaughlin S. T. Differentiation of myoepithelial cells in the developing rat parotid gland. Am J Anat. 1980 Jul;158(3):299–320. doi: 10.1002/aja.1001580306. [DOI] [PubMed] [Google Scholar]
- SHACKLEFORD J. M., KLAPPER E. C. Structure and carbohydrate histochemistry of mammalian salivary glands. Am J Anat. 1962 Jul;111:25–47. doi: 10.1002/aja.1001110104. [DOI] [PubMed] [Google Scholar]
- Tamarin A. Myoepithelium of the rat submaxillary gland. J Ultrastruct Res. 1966 Oct;16(3):320–338. doi: 10.1016/s0022-5320(66)80066-7. [DOI] [PubMed] [Google Scholar]
- Tandler B., Poulsen J. H. Ultrastructure of the cat sublingual gland. Anat Rec. 1977 Feb;187(2):153–171. doi: 10.1002/ar.1091870204. [DOI] [PubMed] [Google Scholar]