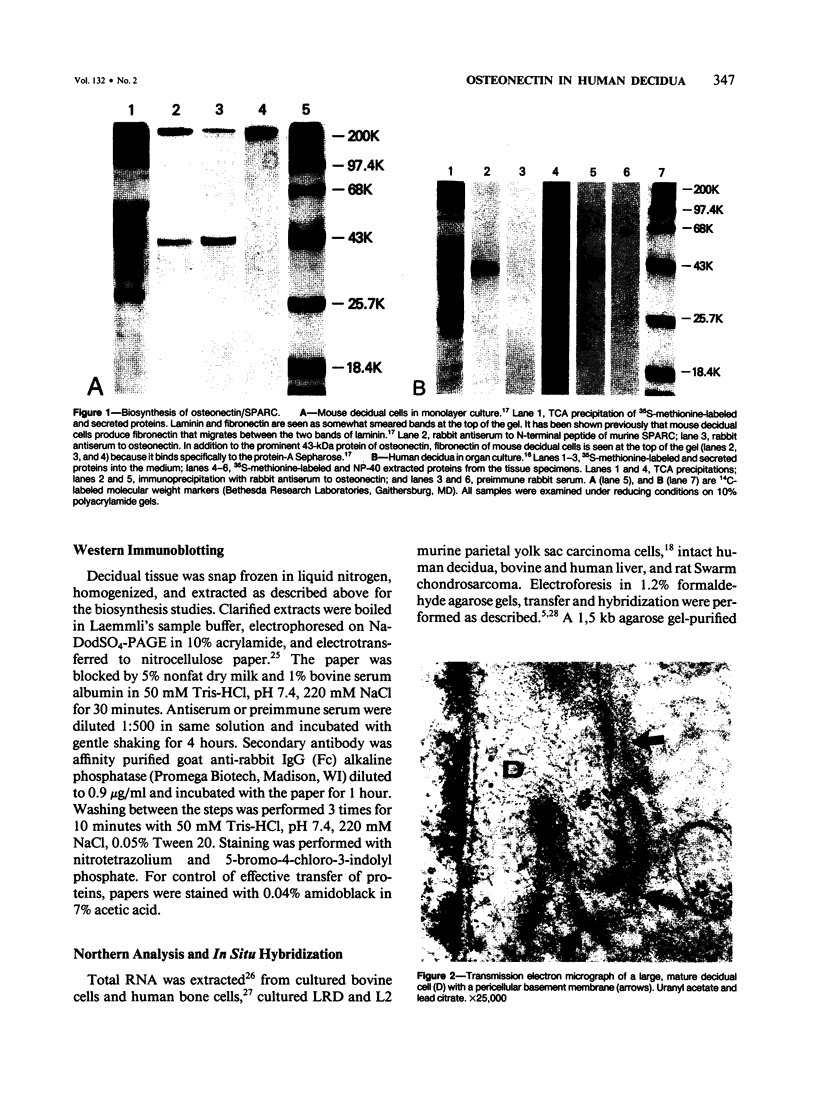
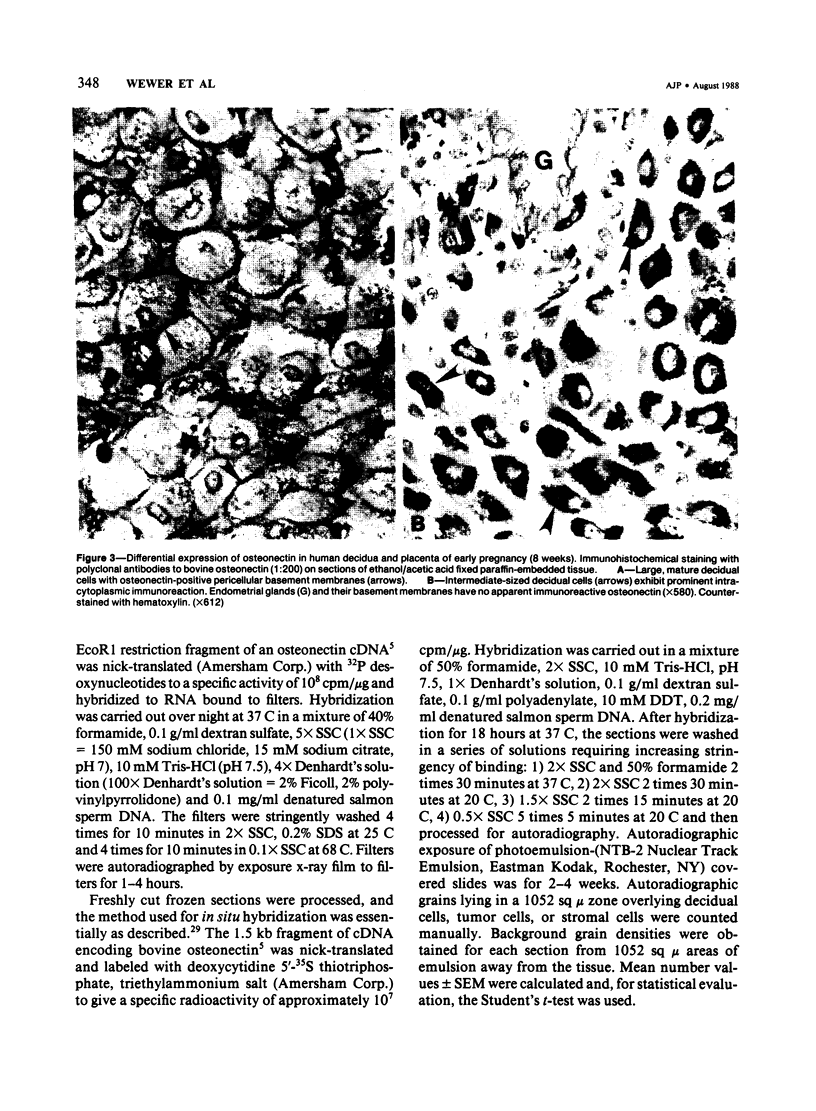
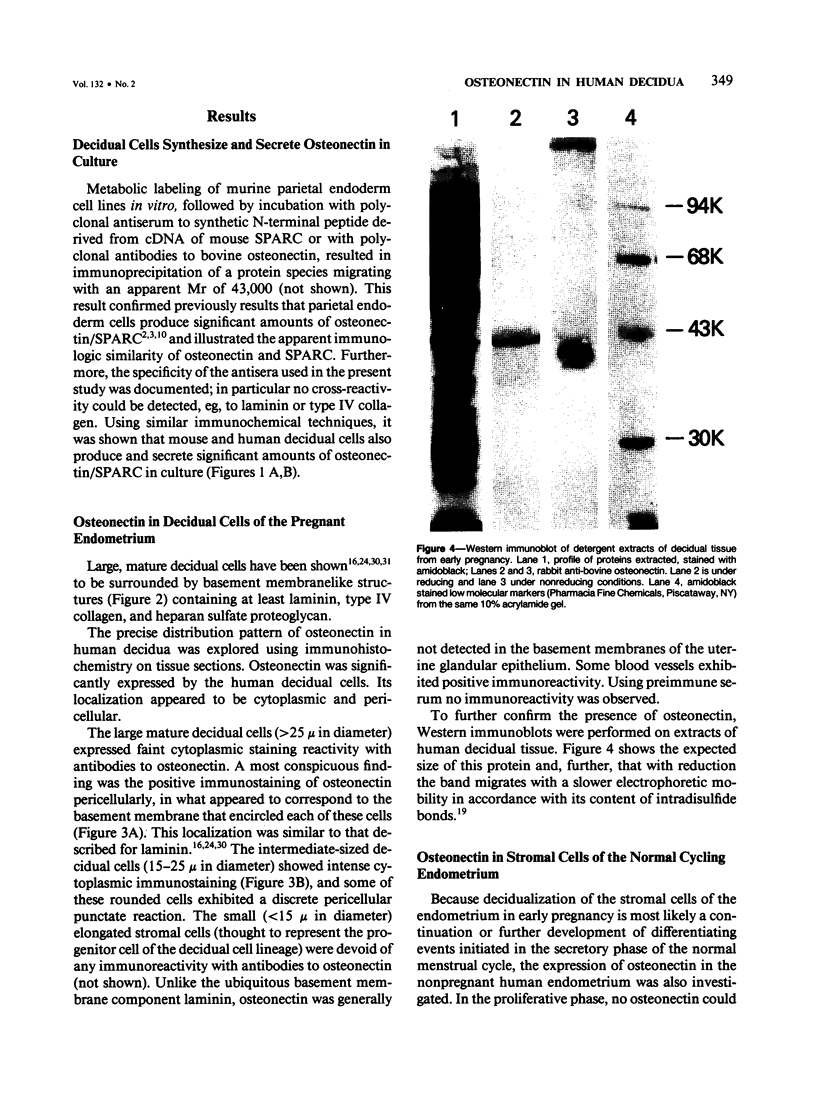
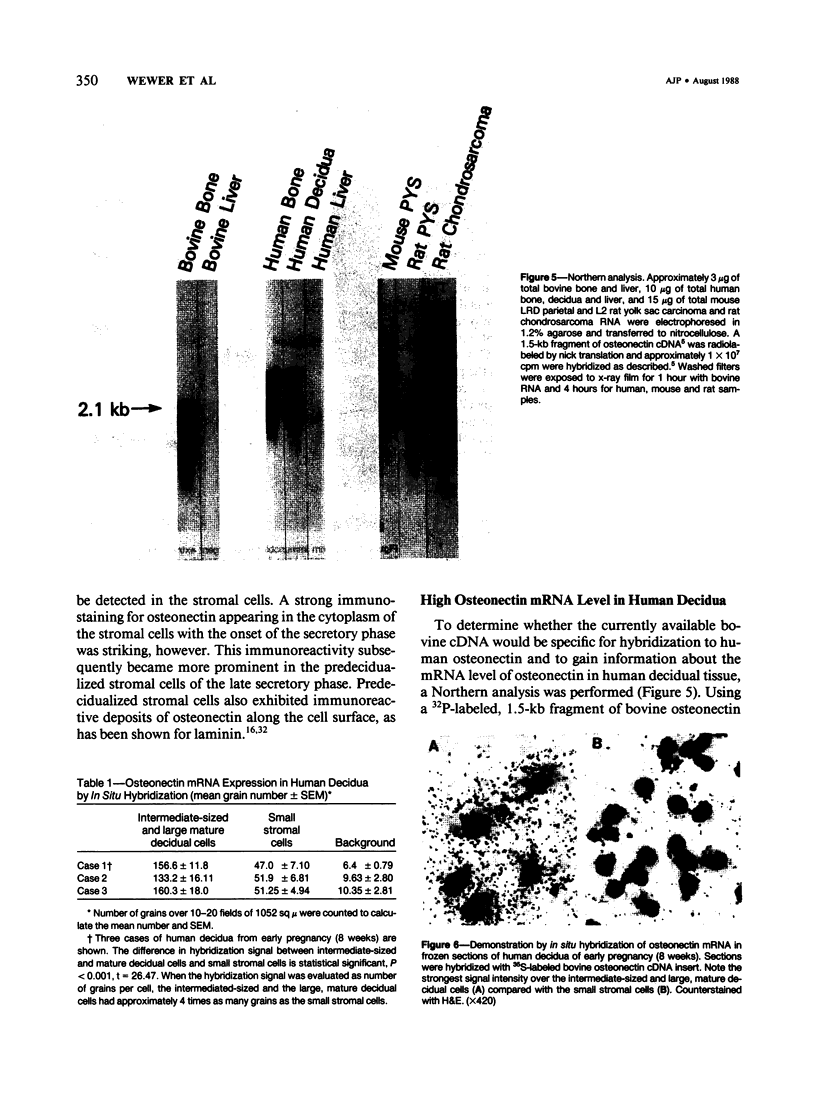
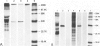Abstract
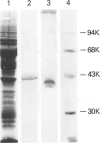
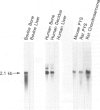
The identification and cDNA cloning of bone-enriched osteonectin and parietal endoderm-enriched SPARC indicated that these proteins share greater than 90% homology. The present study reports substantial expression of this Mr 43,000 protein in human decidua and carcinoma. Metabolic labeling of human decidua tissue in organ culture demonstrated significant osteonectin biosynthesis. Further, Northern analysis and in situ hybridization showed that the osteonectin mRNA level in human decidua is very high. Immunohistochemically, the large mature decidual cells exhibited faint cytoplasmic immunoreactivity with antibodies to osteonectin and a distinct immunoreactivity of the newly deposited basement membranes encircling these cells. The intermediate-sized decidual cells exhibited a strong cytoplasmic immunostaining with antibodies to osteonectin. The small elongated stromal cells were devoid of osteonectin immunoreactivity. Blood vessels exhibited variable positive immunoreactivity. The osteonectin expression in 38 cases of human carcinomas was examined. In well-differentiated carcinomas osteonectin immunoreactivity was located in the basement membrane area, codistributing with laminin. Cytoplasmic osteonectin immunoreactivity was found in poorly differentiated carcinomas. Stromal cells in the peritumoral tissue and some vessels were immunoreactive. Using in situ hybridization, it appeared that both the tumor cells and the stromal cells contained osteonectin transcripts. In normal steady state human nonosseus tissues investigated, osteonectin was found inconsistently in the basement membranes only, as based on the present immunohistochemical technique. These results suggest that the osteonectin/SPARC gene appears activated in certain human tissues characterized by de novo formation of basement membrane.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho S., Tate V., Boedtker H. Multiple 3' ends of the chicken pro alpha 2(I) collagen gene. Nucleic Acids Res. 1983 Aug 25;11(16):5443–5450. doi: 10.1093/nar/11.16.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechtsen R., Nielsen M., Wewer U., Engvall E., Ruoslahti E. Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981 Dec;41(12 Pt 1):5076–5081. [PubMed] [Google Scholar]
- Albrechtsen R., Wewer U. M., Liotta L. A. Basement membranes in human cancer. Pathol Annu. 1986;21(Pt 2):251–276. [PubMed] [Google Scholar]
- Bolander M. E., Young M. F., Fisher L. W., Yamada Y., Termine J. D. Osteonectin cDNA sequence reveals potential binding regions for calcium and hydroxyapatite and shows homologies with both a basement membrane protein (SPARC) and a serine proteinase inhibitor (ovomucoid). Proc Natl Acad Sci U S A. 1988 May;85(9):2919–2923. doi: 10.1073/pnas.85.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Breborowicz J., Tamaoki T. Detection of messenger RNAs of alpha-fetoprotein and albumin in a human hepatoma cell line by in situ hybridization. Cancer Res. 1985 Apr;45(4):1730–1736. [PubMed] [Google Scholar]
- Charpin C., Kopp F., Pourreau-Schneider N., Lissitzky J. C., Lavaut M. N., Martin P. M., Toga M. Laminin distribution in human decidua and immature placenta. An immunoelectron microscopic study (avidin-biotin-peroxidase complex method). Am J Obstet Gynecol. 1985 Mar 15;151(6):822–826. doi: 10.1016/0002-9378(85)90529-0. [DOI] [PubMed] [Google Scholar]
- Damjanov I. Vesalius and Hunter were right: decidua is a membrane! Lab Invest. 1985 Dec;53(6):597–598. [PubMed] [Google Scholar]
- Dziadek M., Paulsson M., Aumailley M., Timpl R. Purification and tissue distribution of a small protein (BM-40) extracted from a basement membrane tumor. Eur J Biochem. 1986 Dec 1;161(2):455–464. doi: 10.1111/j.1432-1033.1986.tb10466.x. [DOI] [PubMed] [Google Scholar]
- Engel J., Taylor W., Paulsson M., Sage H., Hogan B. Calcium binding domains and calcium-induced conformational transition of SPARC/BM-40/osteonectin, an extracellular glycoprotein expressed in mineralized and nonmineralized tissues. Biochemistry. 1987 Nov 3;26(22):6958–6965. doi: 10.1021/bi00396a015. [DOI] [PubMed] [Google Scholar]
- Faber M., Wewer U. M., Berthelsen J. G., Liotta L. A., Albrechtsen R. Laminin production by human endometrial stromal cells relates to the cyclic and pathologic state of the endometrium. Am J Pathol. 1986 Sep;124(3):384–391. [PMC free article] [PubMed] [Google Scholar]
- Findlay D. M., Fisher L. W., McQuillan C. I., Termine J. D., Young M. F. Isolation of the osteonectin gene: evidence that a variable region of the osteonectin molecule is encoded within one exon. Biochemistry. 1988 Mar 8;27(5):1483–1489. doi: 10.1021/bi00405a013. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Hawkins G. R., Tuross N., Termine J. D. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J Biol Chem. 1987 Jul 15;262(20):9702–9708. [PubMed] [Google Scholar]
- Holland P. W., Harper S. J., McVey J. H., Hogan B. L. In vivo expression of mRNA for the Ca++-binding protein SPARC (osteonectin) revealed by in situ hybridization. J Cell Biol. 1987 Jul;105(1):473–482. doi: 10.1083/jcb.105.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jundt G., Berghäuser K. H., Termine J. D., Schulz A. Osteonectin--a differentiation marker of bone cells. Cell Tissue Res. 1987 May;248(2):409–415. doi: 10.1007/BF00218209. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Mann K., Deutzmann R., Paulsson M., Timpl R. Solubilization of protein BM-40 from a basement membrane tumor with chelating agents and evidence for its identity with osteonectin and SPARC. FEBS Lett. 1987 Jun 22;218(1):167–172. doi: 10.1016/0014-5793(87)81040-2. [DOI] [PubMed] [Google Scholar]
- Mason I. J., Murphy D., Münke M., Francke U., Elliott R. W., Hogan B. L. Developmental and transformation-sensitive expression of the Sparc gene on mouse chromosome 11. EMBO J. 1986 Aug;5(8):1831–1837. doi: 10.1002/j.1460-2075.1986.tb04434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I. J., Taylor A., Williams J. G., Sage H., Hogan B. L. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell 'culture shock' glycoprotein of Mr 43,000. EMBO J. 1986 Jul;5(7):1465–1472. doi: 10.1002/j.1460-2075.1986.tb04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey P. G., Termine J. D. Human bone cells in vitro. Calcif Tissue Int. 1985 Sep;37(5):453–460. [PubMed] [Google Scholar]
- Romberg R. W., Werness P. G., Lollar P., Riggs B. L., Mann K. G. Isolation and characterization of native adult osteonectin. J Biol Chem. 1985 Mar 10;260(5):2728–2736. [PubMed] [Google Scholar]
- Sage H., Johnson C., Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem. 1984 Mar 25;259(6):3993–4007. [PubMed] [Google Scholar]
- Sage H., Tupper J., Bramson R. Endothelial cell injury in vitro is associated with increased secretion of an Mr 43,000 glycoprotein ligand. J Cell Physiol. 1986 Jun;127(3):373–387. doi: 10.1002/jcp.1041270305. [DOI] [PubMed] [Google Scholar]
- Seemayer T. A., Lagacé R., Schürch W., Tremblay G. Myofibroblasts in the stroma of invasive and metastatic carcinoma: a possible host response to neoplasia. Am J Surg Pathol. 1979 Dec;3(6):525–533. doi: 10.1097/00000478-197912000-00005. [DOI] [PubMed] [Google Scholar]
- Stenner D. D., Tracy R. P., Riggs B. L., Mann K. G. Human platelets contain and secrete osteonectin, a major protein of mineralized bone. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6892–6896. doi: 10.1073/pnas.83.18.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termine J. D., Belcourt A. B., Conn K. M., Kleinman H. K. Mineral and collagen-binding proteins of fetal calf bone. J Biol Chem. 1981 Oct 25;256(20):10403–10408. [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung P. S., Domenicucci C., Wasi S., Sodek J. Specific immunohistochemical localization of osteonectin and collagen types I and III in fetal and adult porcine dental tissues. J Histochem Cytochem. 1985 Jun;33(6):531–540. doi: 10.1177/33.6.3889139. [DOI] [PubMed] [Google Scholar]
- Wan Y. J., Wu T. C., Chung A. E., Damjanov I. Monoclonal antibodies to laminin reveal the heterogeneity of basement membranes in the developing and adult mouse tissues. J Cell Biol. 1984 Mar;98(3):971–979. doi: 10.1083/jcb.98.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewer U. M., Damjanov A., Weiss J., Liotta L. A., Damjanov I. Mouse endometrial stromal cells produce basement-membrane components. Differentiation. 1986;32(1):49–58. doi: 10.1111/j.1432-0436.1986.tb00555.x. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Faber M., Liotta L. A., Albrechtsen R. Immunochemical and ultrastructural assessment of the nature of the pericellular basement membrane of human decidual cells. Lab Invest. 1985 Dec;53(6):624–633. [PubMed] [Google Scholar]
- Wewer U. M., Tichy D., Damjanov A., Paulsson M., Damjanov I. Distinct antigenic characteristics of murine parietal yolk sac laminin. Dev Biol. 1987 Jun;121(2):397–407. doi: 10.1016/0012-1606(87)90176-x. [DOI] [PubMed] [Google Scholar]
- Young M. F., Bolander M. E., Day A. A., Ramis C. I., Robey P. G., Yamada Y., Termine J. D. Osteonectin mRNA: distribution in normal and transformed cells. Nucleic Acids Res. 1986 Jun 11;14(11):4483–4497. doi: 10.1093/nar/14.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P. D., Tsilibary E. C., Charonis A. S., Furthmayr H. Laminin polymerization in vitro. Evidence for a two-step assembly with domain specificity. J Biol Chem. 1985 Jun 25;260(12):7636–7644. [PubMed] [Google Scholar]