Abstract
Cytokeratins are a set of 19 proteins that together constitute the class of intermediate filament protein expressed by epithelial cells and tumors. Using a panel of 9 different monoclonal anti-cytokeratin antibodies, the authors have performed immunocytochemistry on methanol-fixed, frozen sections and methacarn-fixed, paraffin-embedded tissue of human myometrial specimens. Anomalous cytokeratin expression (ACE) by smooth muscle cells was found in all specimens. Immunoblots of this tissue confirmed the presence of cytokeratin 19, and possibly 8. In addition, immunocytochemical studies demonstrated ACE in human fetal tissues within the intestinal muscularis and the heart, especially in the region of the aortic outflow tract, and in 8 of 19 cases of leiomyosarcoma from adults. Indirect immunofluorescence studies were also performed on cells explanted from myometrial tissue; the overwhelming majority of cells derived from these cultures were smooth muscle cells as verified by expression of muscle actins, and a subpopulation of these cells was found to be cytokeratin-positive. ACE was confirmed in vitro by double labeling experiments demonstrating simultaneous expression of muscle actins and cytokeratins within the same cell. The significance of this smooth muscle cell ACE is unknown, but it may be a phenotypic marker of smooth muscle in a proliferative state. ACE could be a source of confusion in the immunocytochemical analysis of poorly differentiated malignancies if a complete panel of antibodies is not employed.
Full text
PDF




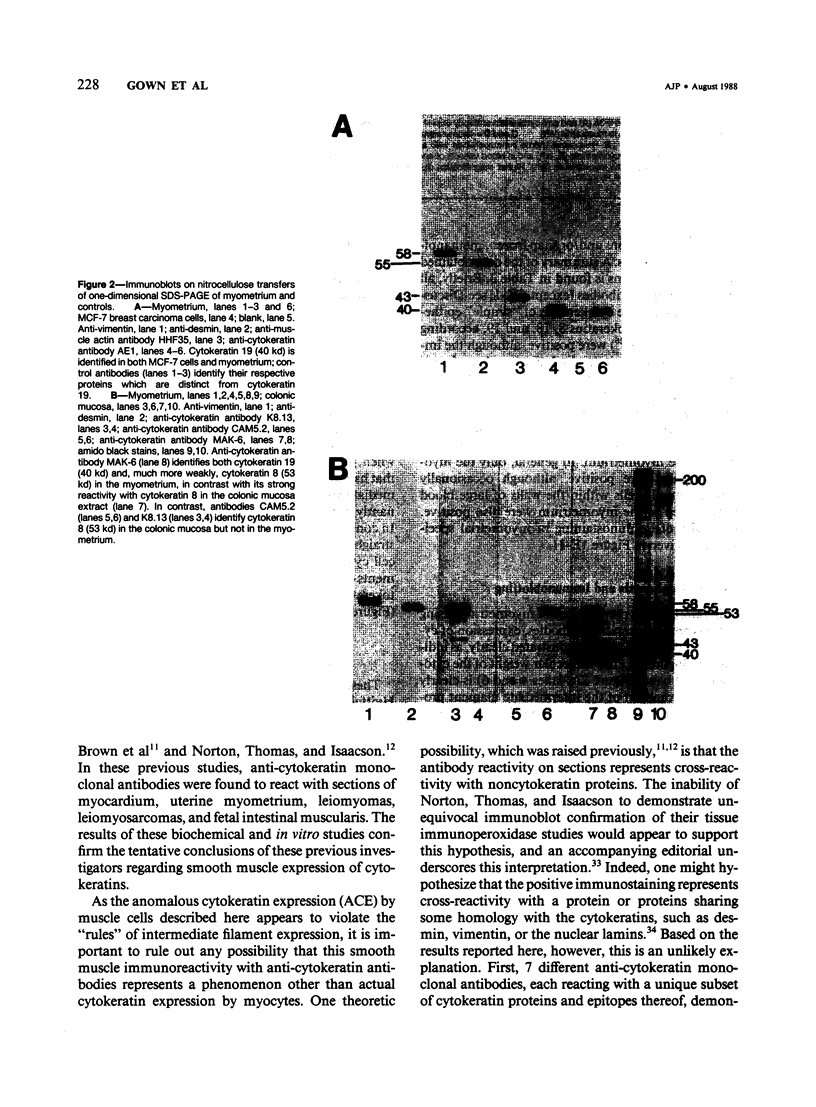




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. C., Theaker J. M., Banks P. M., Gatter K. C., Mason D. Y. Cytokeratin expression in smooth muscle and smooth muscle tumours. Histopathology. 1987 May;11(5):477–486. doi: 10.1111/j.1365-2559.1987.tb02656.x. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Chase D. R., Enzinger F. M., Weiss S. W., Langloss J. M. Keratin in epithelioid sarcoma. An immunohistochemical study. Am J Surg Pathol. 1984 Jun;8(6):435–441. doi: 10.1097/00000478-198406000-00004. [DOI] [PubMed] [Google Scholar]
- Cooper D., Schermer A., Sun T. T. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985 Mar;52(3):243–256. [PubMed] [Google Scholar]
- Cordon-Cardo C., Finstad C. L., Bander N. H., Melamed M. R. Immunoanatomic distribution of cytostructural and tissue-associated antigens in the human urinary tract. Am J Pathol. 1987 Feb;126(2):269–284. [PMC free article] [PubMed] [Google Scholar]
- Dale B. A., Holbrook K. A., Kimball J. R., Hoff M., Sun T. T. Expression of epidermal keratins and filaggrin during human fetal skin development. J Cell Biol. 1985 Oct;101(4):1257–1269. doi: 10.1083/jcb.101.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal antibodies to desmin, the muscle-specific intermediate filament protein. EMBO J. 1983;2(12):2305–2312. doi: 10.1002/j.1460-2075.1983.tb01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal cytokeratin antibodies that distinguish simple from stratified squamous epithelia: characterization on human tissues. EMBO J. 1982;1(12):1641–1647. doi: 10.1002/j.1460-2075.1982.tb01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Grund C., Achtstätter T. Co-expression of cytokeratins and neurofilament proteins in a permanent cell line: cultured rat PC12 cells combine neuronal and epithelial features. J Cell Biol. 1986 Nov;103(5):1933–1943. doi: 10.1083/jcb.103.5.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Moll R., Schiller D. L., Schmid E., Kartenbeck J., Mueller H. Desmoplakins of epithelial and myocardial desmosomes are immunologically and biochemically related. Differentiation. 1982;23(2):115–127. doi: 10.1111/j.1432-0436.1982.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Kapanci Y., Barazzone P., Franke W. W. Immunochemical identification of intermediate-sized filaments in human neoplastic cells. A diagnostic aid for the surgical pathologist. Am J Pathol. 1981 Sep;104(3):206–216. [PMC free article] [PubMed] [Google Scholar]
- Gatter K. C., Ralfkiaer E., Skinner J., Brown D., Heryet A., Pulford K. A., Hou-Jensen K., Mason D. Y. An immunocytochemical study of malignant melanoma and its differential diagnosis from other malignant tumours. J Clin Pathol. 1985 Dec;38(12):1353–1357. doi: 10.1136/jcp.38.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigi O., Geiger B., Eshhar Z., Moll R., Schmid E., Winter S., Schiller D. L., Franke W. W. Detection of a cytokeratin determinant common to diverse epithelial cells by a broadly cross-reacting monoclonal antibody. EMBO J. 1982;1(11):1429–1437. doi: 10.1002/j.1460-2075.1982.tb01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A. E., Maul G., Steinert P. M., Yang H. Y., Goldman R. D. Keratin-like proteins that coisolate with intermediate filaments of BHK-21 cells are nuclear lamins. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3839–3843. doi: 10.1073/pnas.83.11.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Hoak D., Gough F., McNutt M. A. Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol. 1986 May;123(2):195–203. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984 Feb;114(2):309–321. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. III. Analysis of tumors. Am J Clin Pathol. 1985 Oct;84(4):413–424. doi: 10.1093/ajcp/84.4.413. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to intermediate filament proteins of human cells: unique and cross-reacting antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):414–424. doi: 10.1083/jcb.95.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusterson B. A. Is keratin present in smooth muscle? Histopathology. 1987 May;11(5):549–552. doi: 10.1111/j.1365-2559.1987.tb02663.x. [DOI] [PubMed] [Google Scholar]
- Hoefler H., Denk H., Lackinger E., Helleis G., Polak J. M., Heitz P. U. Immunocytochemical demonstration of intermediate filament cytoskeleton proteins in human endocrine tissues and (neuro-) endocrine tumours. Virchows Arch A Pathol Anat Histopathol. 1986;409(5):609–626. doi: 10.1007/BF00713428. [DOI] [PubMed] [Google Scholar]
- Huitfeldt H. S., Brandtzaeg P. Various keratin antibodies produce immunohistochemical staining of human myocardium and myometrium. Histochemistry. 1985;83(5):381–389. doi: 10.1007/BF00509196. [DOI] [PubMed] [Google Scholar]
- Huitfeldt H., Brandtzaeg P. Human heart muscle contains keratin-like material in intercalated discs. Acta Pathol Microbiol Immunol Scand A. 1984 Nov;92(6):481–482. doi: 10.1111/j.1699-0463.1984.tb04431.x. [DOI] [PubMed] [Google Scholar]
- Jones K. H., Kniss D. A. Propidium iodide as a nuclear counterstain for immunofluorescence studies on cells in culture. J Histochem Cytochem. 1987 Jan;35(1):123–125. doi: 10.1177/35.1.2432112. [DOI] [PubMed] [Google Scholar]
- Krohne G., Benavente R. The nuclear lamins. A multigene family of proteins in evolution and differentiation. Exp Cell Res. 1986 Jan;162(1):1–10. doi: 10.1016/0014-4827(86)90421-0. [DOI] [PubMed] [Google Scholar]
- Lansdorp P. M., van der Kwast T. H., de Boer M., Zeijlemaker W. P. Stepwise amplified immunoperoxidase (PAP) staining. I. Cellular morphology in relation to membrane markers. J Histochem Cytochem. 1984 Feb;32(2):172–178. doi: 10.1177/32.2.6198353. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Makin C. A., Bobrow L. G., Bodmer W. F. Monoclonal antibody to cytokeratin for use in routine histopathology. J Clin Pathol. 1984 Sep;37(9):975–983. doi: 10.1136/jcp.37.9.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt M. A., Bolen J. W., Gown A. M., Hammar S. P., Vogel A. M. Coexpression of intermediate filaments in human epithelial neoplasms. Ultrastruct Pathol. 1985;9(1-2):31–43. doi: 10.3109/01913128509055483. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Virtanen I. Keratin in the epithelial-like cells of classical biphasic synovial sarcoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982 Aug;40(2):157–161. doi: 10.1007/BF02932860. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Virtanen I. Monophasic synovial sarcoma of spindle-cell type. Epithelial differentiation as revealed by ultrastructural features, content of prekeratin and binding of peanut agglutinin. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;44(2):187–199. doi: 10.1007/BF02890169. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Virtanen I., Talerman A. Intermediate filament proteins in human testis and testicular germ-cell tumors. Am J Pathol. 1985 Sep;120(3):402–410. [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Lee I., Gould V. E., Berndt R., Roessner A., Franke W. W. Immunocytochemical analysis of Ewing's tumors. Patterns of expression of intermediate filaments and desmosomal proteins indicate cell type heterogeneity and pluripotential differentiation. Am J Pathol. 1987 May;127(2):288–304. [PMC free article] [PubMed] [Google Scholar]
- Mukai M., Torikata C., Iri H., Hanaoka H., Kawai T., Yakumaru K., Shimoda T., Mikata A., Kageyama K. Cellular differentiation of epithelioid sarcoma. An electron-microscopic, enzyme-histochemical, and immunohistochemical study. Am J Pathol. 1985 Apr;119(1):44–56. [PMC free article] [PubMed] [Google Scholar]
- Nagle R. B., Böcker W., Davis J. R., Heid H. W., Kaufmann M., Lucas D. O., Jarasch E. D. Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J Histochem Cytochem. 1986 Jul;34(7):869–881. doi: 10.1177/34.7.2423579. [DOI] [PubMed] [Google Scholar]
- Nakazato Y., Ishizeki J., Takahashi K., Yamaguchi H., Kamei T., Mori T. Localization of S-100 protein and glial fibrillary acidic protein-related antigen in pleomorphic adenoma of the salivary glands. Lab Invest. 1982 Jun;46(6):621–626. [PubMed] [Google Scholar]
- Norton A. J., Thomas J. A., Isaacson P. G. Cytokeratin-specific monoclonal antibodies are reactive with tumours of smooth muscle derivation. An immunocytochemical and biochemical study using antibodies to intermediate filament cytoskeletal proteins. Histopathology. 1987 May;11(5):487–499. doi: 10.1111/j.1365-2559.1987.tb02657.x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Debus E., Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol. 1984 May;34(1):137–143. [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Quinlan R. A., Schiller D. L., Hatzfeld M., Achtstätter T., Moll R., Jorcano J. L., Magin T. M., Franke W. W. Patterns of expression and organization of cytokeratin intermediate filaments. Ann N Y Acad Sci. 1985;455:282–306. doi: 10.1111/j.1749-6632.1985.tb50418.x. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Broers J., Rot M. K., Oostendorp T., Wagenaar S., Vooijs P. Detection of epithelial- and neural type of intermediate filament proteins in human lung tumors. Acta Histochem Suppl. 1987;34:45–56. [PubMed] [Google Scholar]
- Steinberg M. S., Shida H., Giudice G. J., Shida M., Patel N. H., Blaschuk O. W. On the molecular organization, diversity and functions of desmosomal proteins. Ciba Found Symp. 1987;125:3–25. doi: 10.1002/9780470513408.ch2. [DOI] [PubMed] [Google Scholar]
- Tsukada T., McNutt M. A., Ross R., Gown A. M. HHF35, a muscle actin-specific monoclonal antibody. II. Reactivity in normal, reactive, and neoplastic human tissues. Am J Pathol. 1987 May;127(2):389–402. [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Van Muijen G. N., Ruiter D. J., Warnaar S. O. Coexpression of intermediate filament polypeptides in human fetal and adult tissues. Lab Invest. 1987 Oct;57(4):359–369. [PubMed] [Google Scholar]
- Zauli D., Gobbi M., Crespi C., Tazzari P. L., Miserocchi F., Magnani M., Testoni N. Vimentin and keratin intermediate filaments expression by K562 leukemic cell line. Leuk Res. 1986;10(1):29–33. doi: 10.1016/0145-2126(86)90102-5. [DOI] [PubMed] [Google Scholar]