Abstract
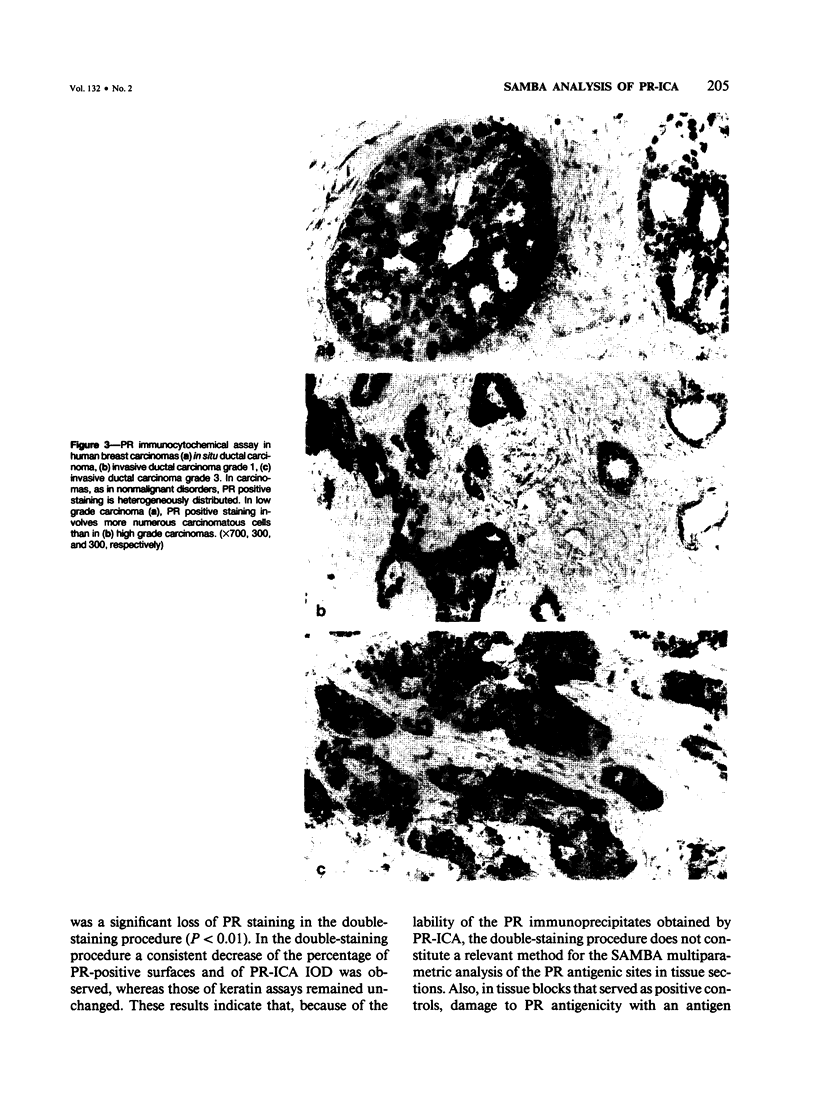
An immunocytochemical assay using monoclonal anti-progesterone receptor (PR-ICA) was performed in nonmalignant (N = 57) and malignant (N = 200) breast disorders. The results were analyzed with a computerized system of image analysis referred to as SAMBA and correlated with binding assays (DCC), and with standard histopathologic findings. It was shown that there was a correlation between 1) the PR-ICA and the binding assays (91.5%), and 2) between the binding assays and the multiparametric computerized (SAMBA) analysis of the PR-ICA. It was also shown that SAMBA provides an accurate, reliable, and reproducible evaluation of PR-ICA that is complementary to binding assays and constitutes a standardized method of evaluating the heterogeneity of the progesterone receptor (PR) distribution in tumors. It is concluded that SAMBA analysis of both the PR-ICA and estrogen receptor (ER-ICA) should improve the prognostic evaluation and the prediction of responsiveness to endocrine therapy in breast carcinomas.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOOM H. J., RICHARDSON W. W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957 Sep;11(3):359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A. J., Sloane J. P., Trickey B. S., Ormerod M. G. An immunocytochemical study of alpha-lactalbumin in human breast tissue. J Pathol. 1982 May;137(1):13–23. doi: 10.1002/path.1711370103. [DOI] [PubMed] [Google Scholar]
- Barsky S. H., Siegal G. P., Jannotta F., Liotta L. A. Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab Invest. 1983 Aug;49(2):140–147. [PubMed] [Google Scholar]
- Boilly B., Moustafa Y., Oudkhir M. Image analysis of blastema cell proliferation in denervated limb regenerates of the newt, Pleurodeles waltlii M. Differentiation. 1986;32(3):208–214. doi: 10.1111/j.1432-0436.1986.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Brugal G., Quirion C., Vassilakos P. Detection of bladder cancers using a SAMBA 200 cell image processor. Anal Quant Cytol Histol. 1986 Sep;8(3):187–194. [PubMed] [Google Scholar]
- Bussolati G., Pich A., Alfani V. Immunofluorescence detection of casein in human mammary dysplastic and neoplastic tissues. Virchows Arch A Pathol Anat Histol. 1975;365(1):15–21. doi: 10.1007/BF00439282. [DOI] [PubMed] [Google Scholar]
- Charpin C., Bhan A. K., Zurawski V. R., Jr, Scully R. E. Carcinoembryonic antigen (CEA) and carbohydrate determinant 19-9 (CA 19-9) localization in 121 primary and metastatic ovarian tumors: an immunohistochemical study with the use of monoclonal antibodies. Int J Gynecol Pathol. 1982;1(3):231–245. doi: 10.1097/00004347-198203000-00001. [DOI] [PubMed] [Google Scholar]
- Charpin C., Lachard A., Pourreau-Schneider N., Jacquemier J., Lavaut M. N., Andonian C., Martin P. M., Toga M. Localization of lactoferrin and nonspecific cross-reacting antigen in human breast carcinomas. An immunohistochemical study using the avidin-biotin-peroxidase complex method. Cancer. 1985 Jun 1;55(11):2612–2617. doi: 10.1002/1097-0142(19850601)55:11<2612::aid-cncr2820551113>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Charpin C., Lissitzky J. C., Jacquemier J., Lavaut M. N., Kopp F., Pourreau-Schneider N., Martin P. M., Toga M. Immunohistochemical detection of laminin in 98 human breast carcinomas: a light and electron microscopic study. Hum Pathol. 1986 Apr;17(4):355–365. doi: 10.1016/s0046-8177(86)80458-0. [DOI] [PubMed] [Google Scholar]
- Charpin C., Martin P. M., De Victor B., Lavaut M. N., Habib M. C., Andrac L., Toga M. Multiparametric study (SAMBA 200) of estrogen receptor immunocytochemical assay in 400 human breast carcinomas: analysis of estrogen receptor distribution heterogeneity in tissues and correlations with dextran coated charcoal assays and morphological data. Cancer Res. 1988 Mar 15;48(6):1578–1586. [PubMed] [Google Scholar]
- Charpin C., Martin P. M., Lachard A., Jacquemier J., Lavaut M. N., Andonian C., Pourreau-Schneider N., Toga M. Kappa casein, lactalbumin and GCDFP 70 localization in human breast carcinomas: an immunohistochemical study using the avidin-biotin-peroxidase complex method. Med Oncol Tumor Pharmacother. 1985;2(2):103–112. doi: 10.1007/BF02934856. [DOI] [PubMed] [Google Scholar]
- Charpin C., Martin P. M., Lavaut M. N., Pourreau-Schneider N., Toga M. Estrogen receptor immunocytochemical assay (ER-ICA) in human endometrium. Int J Gynecol Pathol. 1986;5(2):119–131. [PubMed] [Google Scholar]
- Charpin C., Martin P. M., Lissitzky J. C., Jacquemier J., Kopp F., Pourreau-Schneider N., Lavaut M. N., Toga M. Estrogen receptor immunocytochemical assay (ERICA) and laminin detection in 130 breast carcinomas and computerized (Samba 200) multiparametric quantitative analysis on tissue sections. Bull Cancer. 1986;73(6):651–664. [PubMed] [Google Scholar]
- Clayton F., Ordóez N. G., Hanssen G. M., Hanssen H. Immunoperoxidase localization of lactalbumin in malignant breast neoplasms. Arch Pathol Lab Med. 1982 Jun;106(6):268–270. [PubMed] [Google Scholar]
- Fortt R. W., Gibbs A. R., Williams D., Hansen J., Williams I. The identification of 'casein' in human breast cancer. Histopathology. 1979 Sep;3(5):395–406. doi: 10.1111/j.1365-2559.1979.tb03021.x. [DOI] [PubMed] [Google Scholar]
- Foster C. S., Neville A. M. Monoclonal antibodies to the human mammary gland: III. Monoclonal antibody LICR-LON-M18 identifies impaired expression and excess sialylation of the I(Ma) cell-surface antigen by primary breast carcinoma cells. Hum Pathol. 1984 Jun;15(6):502–513. doi: 10.1016/s0046-8177(84)80003-9. [DOI] [PubMed] [Google Scholar]
- Gusterson B. A., Warburton M. J., Mitchell D., Ellison M., Neville A. M., Rudland P. S. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982 Nov;42(11):4763–4770. [PubMed] [Google Scholar]
- Howell A., Harland R. N., Barnes D. M., Hayward E., Redford J., Swindell R., Sellwood R. A. Endocrine therapy for advanced carcinoma of the breast: effect of tumor heterogeneity and site of biopsy on the predictive value of progesterone receptor estimations. Cancer Res. 1987 Jan 1;47(1):296–299. [PubMed] [Google Scholar]
- King W. J., DeSombre E. R., Jensen E. V., Greene G. L. Comparison of immunocytochemical and steroid-binding assays for estrogen receptor in human breast tumors. Cancer Res. 1985 Jan;45(1):293–304. [PubMed] [Google Scholar]
- Knight W. A., Livingston R. B., Gregory E. J., McGuire W. L. Estrogen receptor as an independent prognostic factor for early recurrence in breast cancer. Cancer Res. 1977 Dec;37(12):4669–4671. [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases: role of the basement membrane. Warner-Lambert Parke-Davis Award lecture. Am J Pathol. 1984 Dec;117(3):339–348. [PMC free article] [PubMed] [Google Scholar]
- Martin P. M., Rolland P. H., Jacquemier J., Rolland A. M., Toga M. Multiple steroid receptors in human breast cancer. III. Relationships between steroid receptors and the state of differentiation and the activity of carcinomas throughout the pathologic features. Cancer Chemother Pharmacol. 1979;2(2):115–120. doi: 10.1007/BF00254083. [DOI] [PubMed] [Google Scholar]
- Mazoujian G., Pinkus G. S., Davis S., Haagensen D. E., Jr Immunohistochemistry of a gross cystic disease fluid protein (GCDFP-15) of the breast. A marker of apocrine epithelium and breast carcinomas with apocrine features. Am J Pathol. 1983 Feb;110(2):105–112. [PMC free article] [PubMed] [Google Scholar]
- McGuire W. L. Steroid hormone receptors in breast cancer treatment strategy. Recent Prog Horm Res. 1980;36:135–156. doi: 10.1016/b978-0-12-571136-4.50010-3. [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M., Groyer-Picard M. T., Lorenzo F., Jolivet A., Vu Hai M. T., Pallud C., Spyratos F., Milgrom E. Immunocytochemical study with monoclonal antibodies to progesterone receptor in human breast tumors. Cancer Res. 1987 May 15;47(10):2652–2661. [PubMed] [Google Scholar]
- Pertschuk L. P., Eisenberg K. B., Carter A. C., Feldman J. G. Heterogeneity of estrogen binding sites in breast cancer: morphologic demonstration and relationship to endocrine response. Breast Cancer Res Treat. 1985;5(2):137–147. doi: 10.1007/BF01805987. [DOI] [PubMed] [Google Scholar]
- Pertschuk L. P., Eisenberg K. B., Carter A. C., Feldman J. G. Immunohistologic localization of estrogen receptors in breast cancer with monoclonal antibodies. Correlation with biochemistry and clinical endocrine response. Cancer. 1985 Apr 1;55(7):1513–1518. doi: 10.1002/1097-0142(19850401)55:7<1513::aid-cncr2820550717>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Pichon M. F., Pallud C., Brunet M., Milgrom E. Relationship of presence of progesterone receptors to prognosis in early breast cancer. Cancer Res. 1980 Sep;40(9):3357–3360. [PubMed] [Google Scholar]
- Seigneurin D., Brugal G., Payard D., Gauvain C. A quantitative analysis of the human bone marrow granulocytic cell lineage using the SAMBA 200 cell image processor. II. The blast cells in refractory anemia with an excess of blasts. Anal Quant Cytol Histol. 1986 Dec;8(4):281–292. [PubMed] [Google Scholar]
- Seigneurin D., Gauvain C., Brugal G. A quantitative analysis of the human bone marrow granulocytic cell lineage using the SAMBA 200 cell image processor. I. The normal maturation sequence. Anal Quant Cytol. 1984 Sep;6(3):168–178. [PubMed] [Google Scholar]
- Sklarew R. J. Simultaneous Feulgen densitometry and autoradiographic grain counting with the Quantimet 720D image-analysis system. I. Estimation of nuclear DNA content in 3H-thymidine-labeled cells. J Histochem Cytochem. 1982 Jan;30(1):35–48. doi: 10.1177/30.1.7054272. [DOI] [PubMed] [Google Scholar]
- Sklarew R. J. Simultaneous Feulgen densitometry and autoradiographic grain counting with the Quantimet 720D image-analysis system. II. Automated grain counting. J Histochem Cytochem. 1982 Jan;30(1):49–57. doi: 10.1177/30.1.7054273. [DOI] [PubMed] [Google Scholar]
- Sklarew R. J. Simultaneous Feulgen densitometry and autoradiographic grain counting with the Quantimet 720D image-analysis system. III. Improvements in Feulgen densitometry. J Histochem Cytochem. 1983 Oct;31(10):1224–1232. doi: 10.1177/31.10.6886380. [DOI] [PubMed] [Google Scholar]
- Walker R. A. Demonstration of carcinoembryonic antigen in human breast carcinomas by the immunoperoxidase technique. J Clin Pathol. 1980 Apr;33(4):356–360. doi: 10.1136/jcp.33.4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. A. The demonstration of alpha lactalbumin in human breast carcinomas. J Pathol. 1979 Sep;129(1):37–42. doi: 10.1002/path.1711290107. [DOI] [PubMed] [Google Scholar]