Abstract
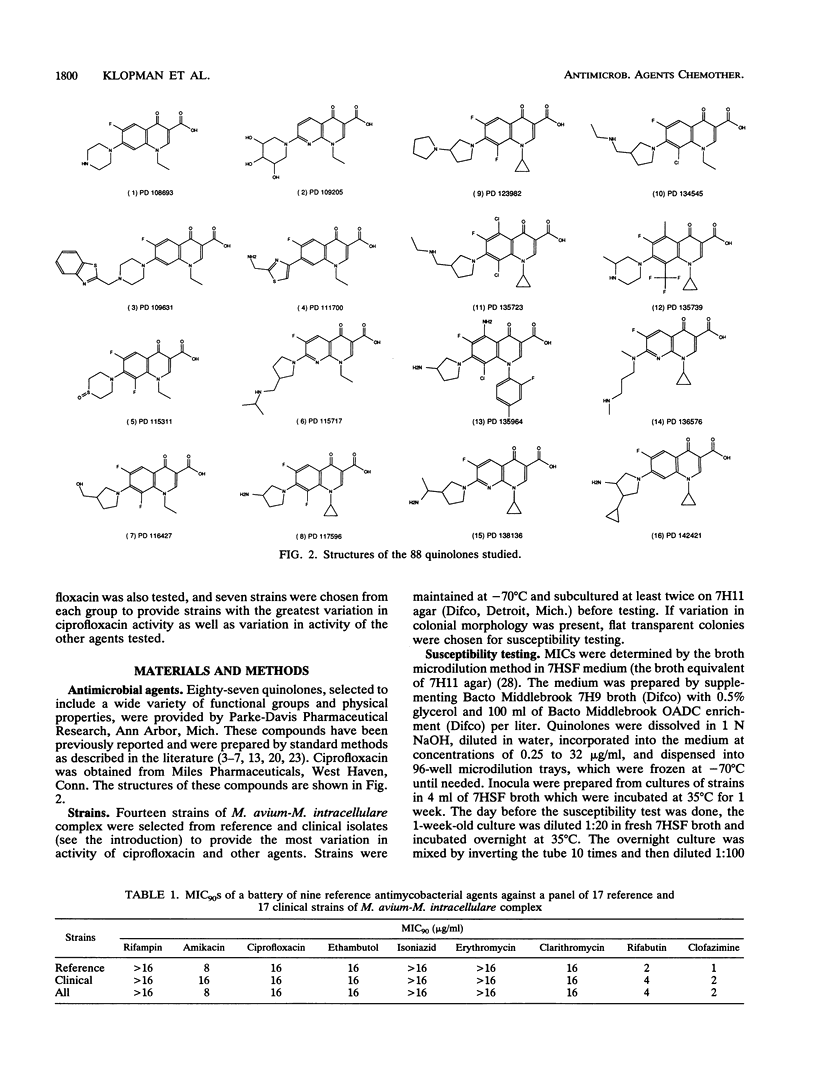
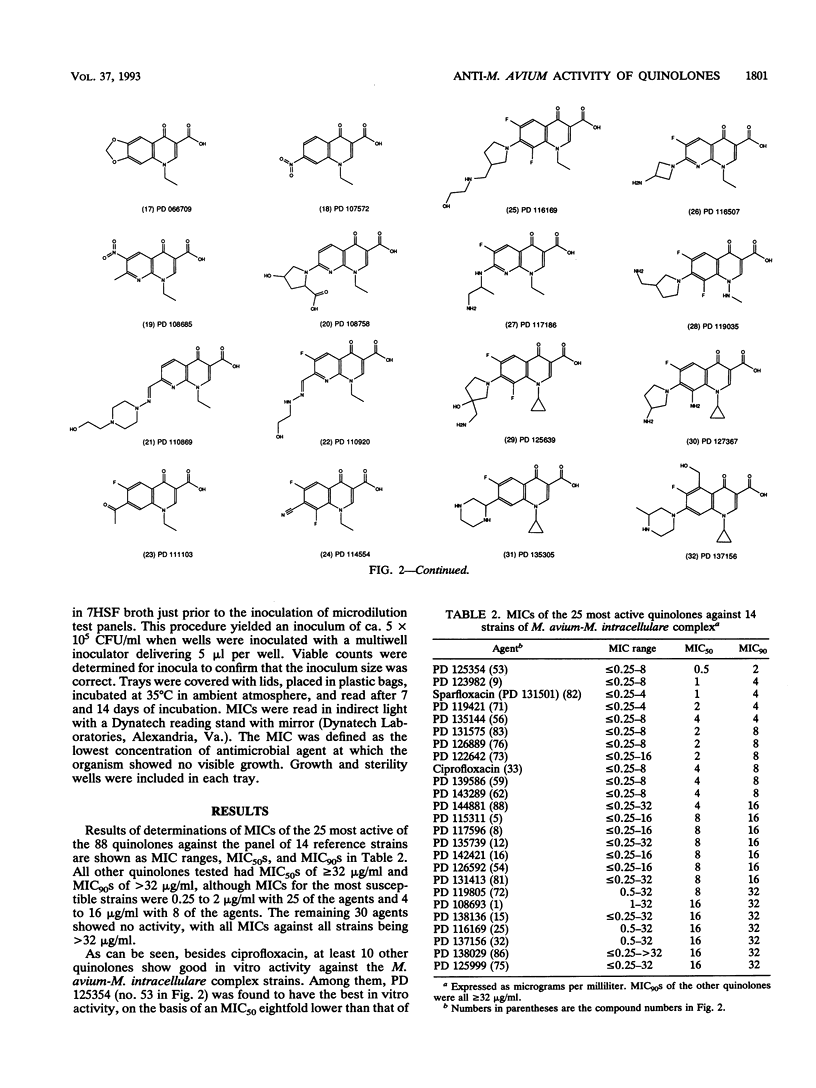
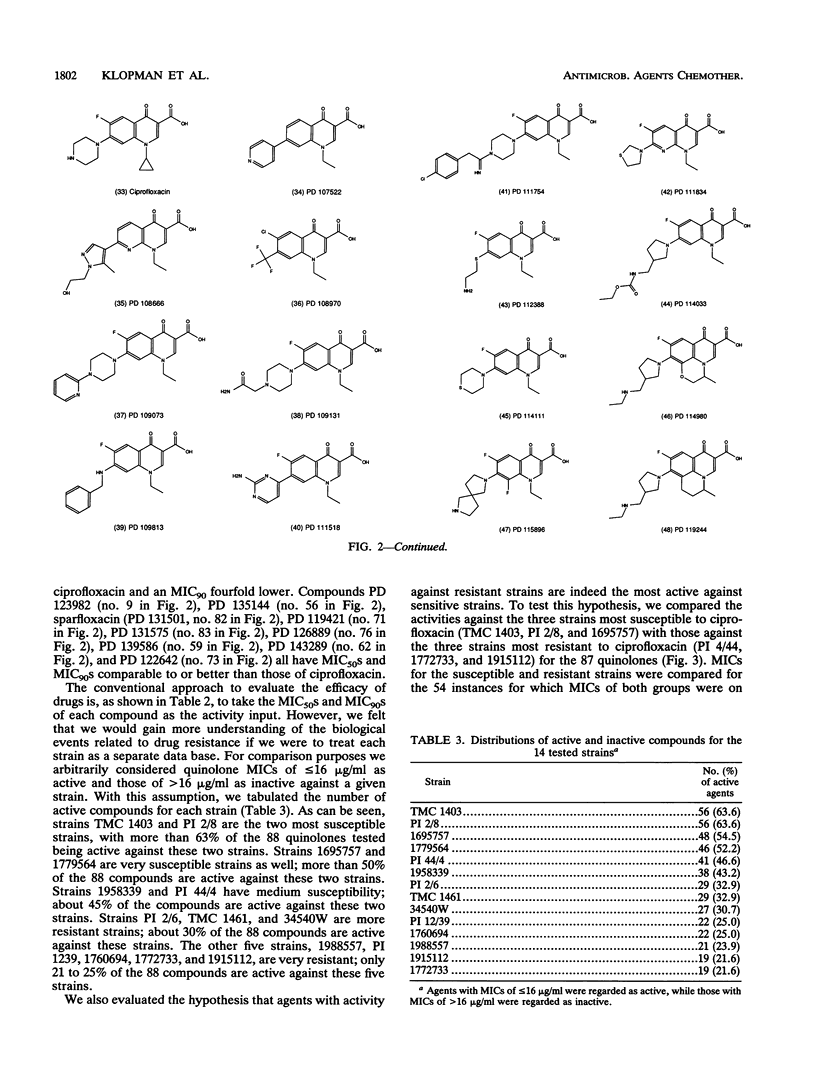
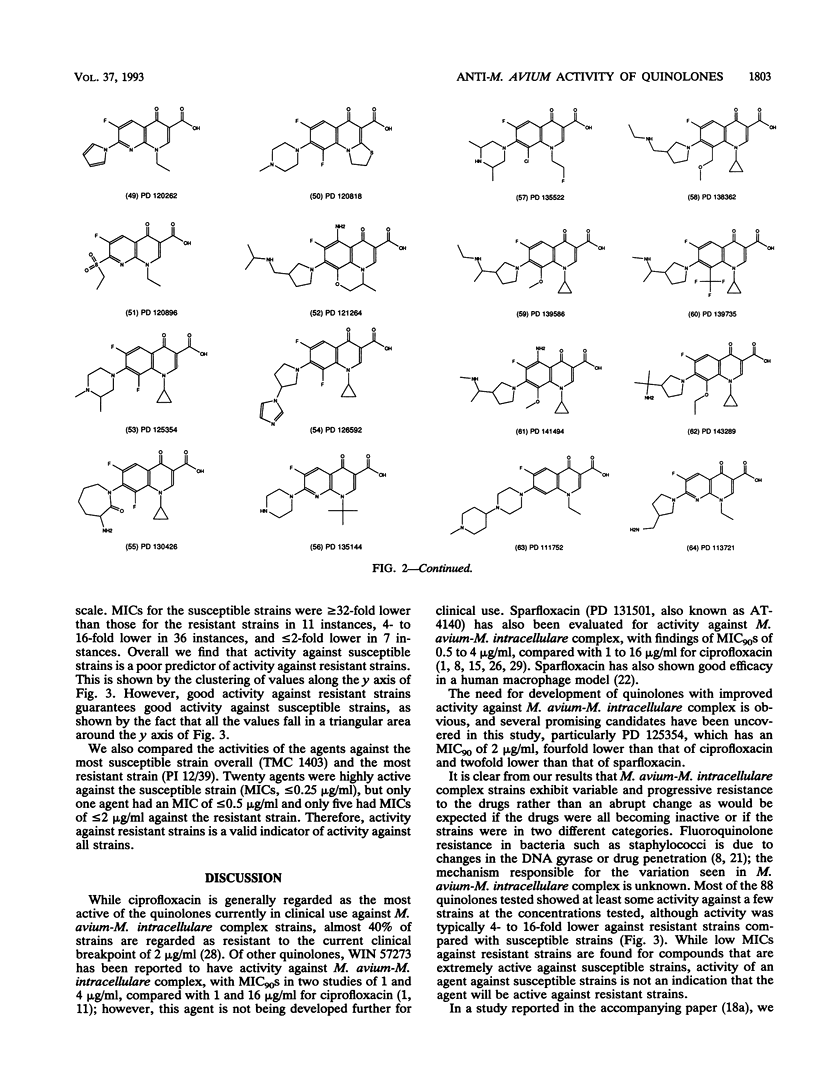
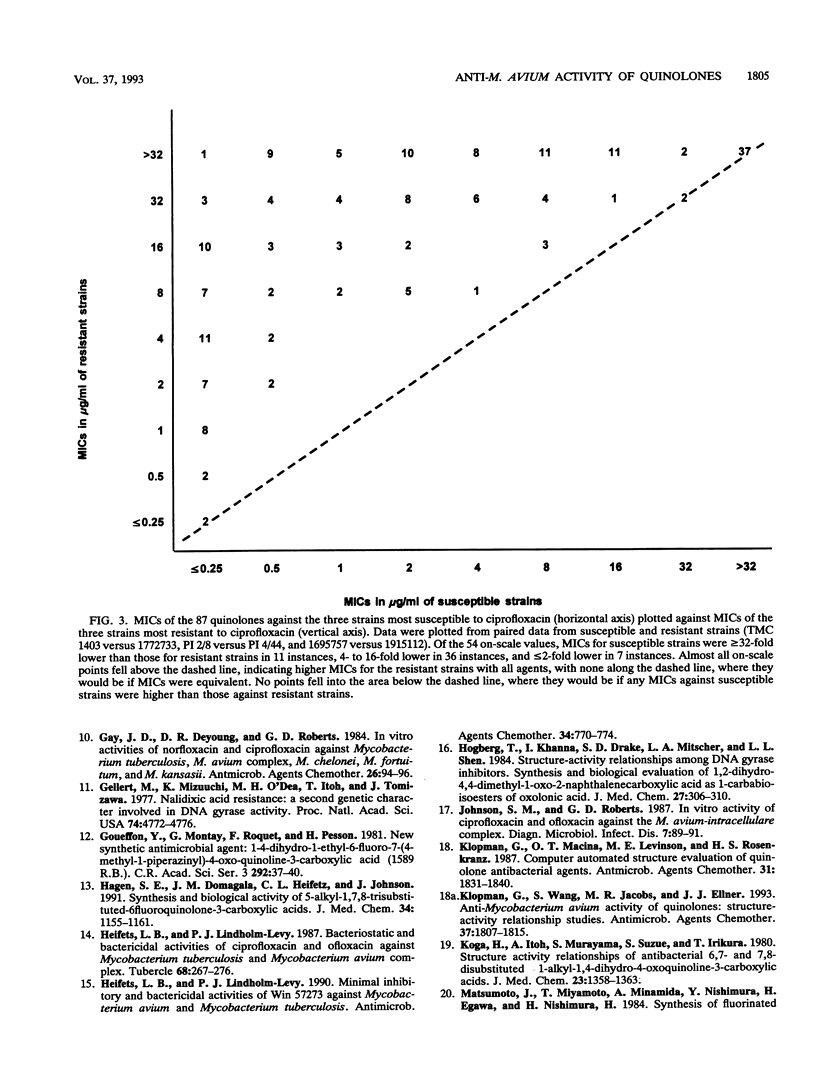
The MICs of 88 quinolones against 14 selected reference and clinical strains of Mycobacterium avium-M. intracellular complex were determined. Agents tested included ciprofloxacin, sparfloxacin (PD 131501), and 86 other experimental quinolones. Test strains were selected to represent various susceptibilities to ciprofloxacin and other drug resistance profiles. MICs were determined by the microdilution method in 7HSF broth, with incubation for 14 days at 35 degrees C. The results showed 25 of the quinolones to be active against the strains, with MICs for 90% of the strains (MIC90s) of 2 to 32 micrograms/ml. Ten of these compounds had activities equivalent to or greater than that of ciprofloxacin. The most active compound was PD 125354, with an MIC50 of 0.5 micrograms/ml and an MIC90 of 2 micrograms/ml; comparable values for ciprofloxacin were 4 and 8 micrograms/ml, respectively. The next most active compounds, with MIC90s of 4 micrograms/ml, were sparfloxacin (PD 131501), PD 123982, PD 135144, and PD 119421. MIC90s of PD 131575, PD 126889, PD 122642, PD 139586, and PD 143289 were 8 micrograms/ml. Further evaluation of the most active agents is warranted, as is assessment of structure-activity relationships of active and inactive agents to elucidate the active portions of the compounds and to lead to the development of compounds with enhanced activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett M. S., Jones R. N., Erwin M. E., Koontz F. P. CI-960 (PD127391 or AM-1091), sparfloxacin, WIN 57273, and isepamicin activity against clinical isolates of Mycobacterium avium-intracellularae complex, M. chelonae, and M. fortuitum. Diagn Microbiol Infect Dis. 1992 Feb;15(2):169–171. doi: 10.1016/0732-8893(92)90044-t. [DOI] [PubMed] [Google Scholar]
- Benbrook D. M., Miller R. V. Effects of norfloxacin on DNA metabolism in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1986 Jan;29(1):1–6. doi: 10.1128/aac.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagala J. M., Bridges A. J., Culbertson T. P., Gambino L., Hagen S. E., Karrick G., Porter K., Sanchez J. P., Sesnie J. A., Spense F. G. Synthesis and biological activity of 5-amino- and 5-hydroxyquinolones, and the overwhelming influence of the remote N1-substituent in determining the structure-activity relationship. J Med Chem. 1991 Mar;34(3):1142–1154. doi: 10.1021/jm00107a039. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Hagen S. E., Joannides T., Kiely J. S., Laborde E., Schroeder M. C., Sesnie J. A., Shapiro M. A., Suto M. J., Vanderroest S. Quinolone antibacterials containing the new 7-[3-(1-aminoethyl)-1- pyrrolidinyl] side chain: the effects of the 1-aminoethyl moiety and its stereochemical configurations on potency and in vivo efficacy. J Med Chem. 1993 Apr 2;36(7):871–882. doi: 10.1021/jm00059a012. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Hanna L. D., Heifetz C. L., Hutt M. P., Mich T. F., Sanchez J. P., Solomon M. New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay. J Med Chem. 1986 Mar;29(3):394–404. doi: 10.1021/jm00153a015. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Klimm K., Grayson M. L. In vitro activity of sparfloxacin (AT-4140, CI-978, PD 131501), a new quinolone antimicrobial agent. Diagn Microbiol Infect Dis. 1990 Jul-Aug;13(4):345–348. doi: 10.1016/0732-8893(90)90029-u. [DOI] [PubMed] [Google Scholar]
- Fenlon C. H., Cynamon M. H. Comparative in vitro activities of ciprofloxacin and other 4-quinolones against Mycobacterium tuberculosis and Mycobacterium intracellulare. Antimicrob Agents Chemother. 1986 Mar;29(3):386–388. doi: 10.1128/aac.29.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay J. D., DeYoung D. R., Roberts G. D. In vitro activities of norfloxacin and ciprofloxacin against Mycobacterium tuberculosis, M. avium complex, M. chelonei, M. fortuitum, and M. kansasii. Antimicrob Agents Chemother. 1984 Jul;26(1):94–96. doi: 10.1128/aac.26.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goueffon Y., Montay G., Roquet F., Pesson M. Sur un nouvel antibactérien de synthèse : l'acide éthyl-1 fluoro-6 (méthyl-4 pipérazinyl-1)-7 oxo-4 dihydro-1.4 quinoléine-3 carboxylique (1589 R.B.). C R Seances Acad Sci III. 1981 Jan 5;292(1):37–40. [PubMed] [Google Scholar]
- Hagen S. E., Domagala J. M., Heifetz C. L., Johnson J. Synthesis and biological activity of 5-alkyl-1,7,8-trisubstituted-6-fluoroquinoline-3-carboxylic acids. J Med Chem. 1991 Mar;34(3):1155–1161. doi: 10.1021/jm00107a040. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Lindholm-Levy P. J. Bacteriostatic and bactericidal activity of ciprofloxacin and ofloxacin against Mycobacterium tuberculosis and Mycobacterium avium complex. Tubercle. 1987 Dec;68(4):267–276. doi: 10.1016/0041-3879(87)90067-5. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Lindholm-Levy P. J. MICs and MBCs of Win 57273 against Mycobacterium avium and M. tuberculosis. Antimicrob Agents Chemother. 1990 May;34(5):770–774. doi: 10.1128/aac.34.5.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högberg T., Khanna I., Drake S. D., Mitscher L. A., Shen L. L. Structure-activity relationships among DNA gyrase inhibitors. Synthesis and biological evaluation of 1,2-dihydro-4, 4-dimethyl-1-oxo-2-naphthalenecarboxylic acids as 1-carba bioisosteres of oxolinic acid. J Med Chem. 1984 Mar;27(3):306–310. doi: 10.1021/jm00369a013. [DOI] [PubMed] [Google Scholar]
- Johnson S. M., Roberts G. D. In vitro activity of ciprofloxacin and ofloxacin against the Mycobacterium avium-intracellulare complex. Diagn Microbiol Infect Dis. 1987 May;7(1):89–91. doi: 10.1016/0732-8893(87)90077-0. [DOI] [PubMed] [Google Scholar]
- Klopman G., Macina O. T., Levinson M. E., Rosenkranz H. S. Computer automated structure evaluation of quinolone antibacterial agents. Antimicrob Agents Chemother. 1987 Nov;31(11):1831–1840. doi: 10.1128/aac.31.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopman G., Wang S., Jacobs M. R., Ellner J. J. Anti-Mycobacterium avium activity of quinolones: structure-activity relationship studies. Antimicrob Agents Chemother. 1993 Sep;37(9):1807–1815. doi: 10.1128/aac.37.9.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H., Itoh A., Murayama S., Suzue S., Irikura T. Structure-activity relationships of antibacterial 6,7- and 7,8-disubstituted 1-alkyl-1,4-dihydro-4-oxoquinoline-3-carboxylic acids. J Med Chem. 1980 Dec;23(12):1358–1363. doi: 10.1021/jm00186a014. [DOI] [PubMed] [Google Scholar]
- Perronne C., Gikas A., Truffot-Pernot C., Grosset J., Vilde J. L., Pocidalo J. J. Activities of sparfloxacin, azithromycin, temafloxacin, and rifapentine compared with that of clarithromycin against multiplication of Mycobacterium avium complex within human macrophages. Antimicrob Agents Chemother. 1991 Jul;35(7):1356–1359. doi: 10.1128/aac.35.7.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J. P., Bridges A. J., Bucsh R., Domagala J. M., Gogliotti R. D., Hagen S. E., Heifetz C. L., Joannides E. T., Sesnie J. C., Shapiro M. A. New 8-(trifluoromethyl)-substituted quinolones. The benefits of the 8-fluoro group with reduced phototoxic risk. J Med Chem. 1992 Jan 24;35(2):361–367. doi: 10.1021/jm00080a023. [DOI] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Inoue M., Une T., Osada Y., Ogawa H., Mitsuhashi S. In vitro and in vivo activity of DL-8280, a new oxazine derivative. Antimicrob Agents Chemother. 1982 Oct;22(4):548–553. doi: 10.1128/aac.22.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Edwards L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983 Apr;23(4):559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Hadley W. K. Broth microdilution testing of susceptibilities to 30 antimicrobial agents of Mycobacterium avium strains from patients with acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1987 Oct;31(10):1579–1584. doi: 10.1128/aac.31.10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Sanders C. A., Nassos P. S., Hadley W. K. In vitro susceptibility of Mycobacterium avium complex to the new fluoroquinolone sparfloxacin (CI-978; AT-4140) and comparison with ciprofloxacin. Antimicrob Agents Chemother. 1990 Dec;34(12):2442–2444. doi: 10.1128/aac.34.12.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Furutani Y., Inoue S., Ohue T., Nakamura S., Shimizu M. New nalidixic acid resistance mutations related to deoxyribonucleic acid gyrase activity. J Bacteriol. 1981 Nov;148(2):450–458. doi: 10.1128/jb.148.2.450-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Berlin O. G., Inderlied C. B. Activity of ciprofloxacin and other fluorinated quinolones against mycobacteria. Am J Med. 1987 Apr 27;82(4A):23–26. [PubMed] [Google Scholar]
- Zweerink M. M., Edison A. Inhibition of Micrococcus luteus DNA gyrase by norfloxacin and 10 other quinolone carboxylic acids. Antimicrob Agents Chemother. 1986 Apr;29(4):598–601. doi: 10.1128/aac.29.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]



