Abstract
The BioBreeding/Worcester (BB/Wor) rat develops a spontaneous disorder that closely resembles human insulin-dependent (Type I) diabetes mellitus. The syndrome is preceded by lymphocytic insulitis that destroys pancreatic beta cells. The morphologic features of the spontaneous insulitis lesions are also observed within islets transplanted beneath the renal capsule of diabetes-prone and diabetic animals. This study reports the results of experiments in which immunohistochemical techniques were used to characterize the phenotype of the infiltrating mononuclear cells and detect the expression of class I and class II MHC antigens in native islets and islet transplants in diabetic and diabetes-prone BB/Wor rats. The infiltrates within native pancreatic islets and islet grafts were comprised predominantly of Ia+ cells (dendritic cells and macrophages) CD4+ cells (helper/inducer lymphocytes and macrophages), CD5+ (pan-T) cells and smaller numbers of CD8+ (cytotoxic/suppressor and NK) cells. Pancreatic and graft insulitis were accompanied by markedly enhanced class I antigen expression on islet and exocrine cells. Class II (Ia) antigens were not detected on normal islet cells, islets undergoing insulitis or on islet transplants subjected to immune attack. In islet grafts stained with polymorphic MAbs that distinguish Ia antigens of donor and host origin, Ia antigen expression was limited to infiltrating dendritic cells and macrophages of host origin. It is concluded that the phenotypes of infiltrating mononuclear cells that comprise the insulitis lesion in spontaneous BB/Wor diabetes, and the inflammatory attack on islets transplanted into diabetic BB/Wor rats are the same, that pancreatic islet and graft insulitis occur in the presence of enhanced class I antigen expression but in the absence of class II antigen expression, and that infiltrating Ia+ cells within islet grafts are exclusively of recipient (BB/Wor) origin and may explain the initiation of immune insulitis within grafts derived from donors of incompatible MHC.
Full text
PDF


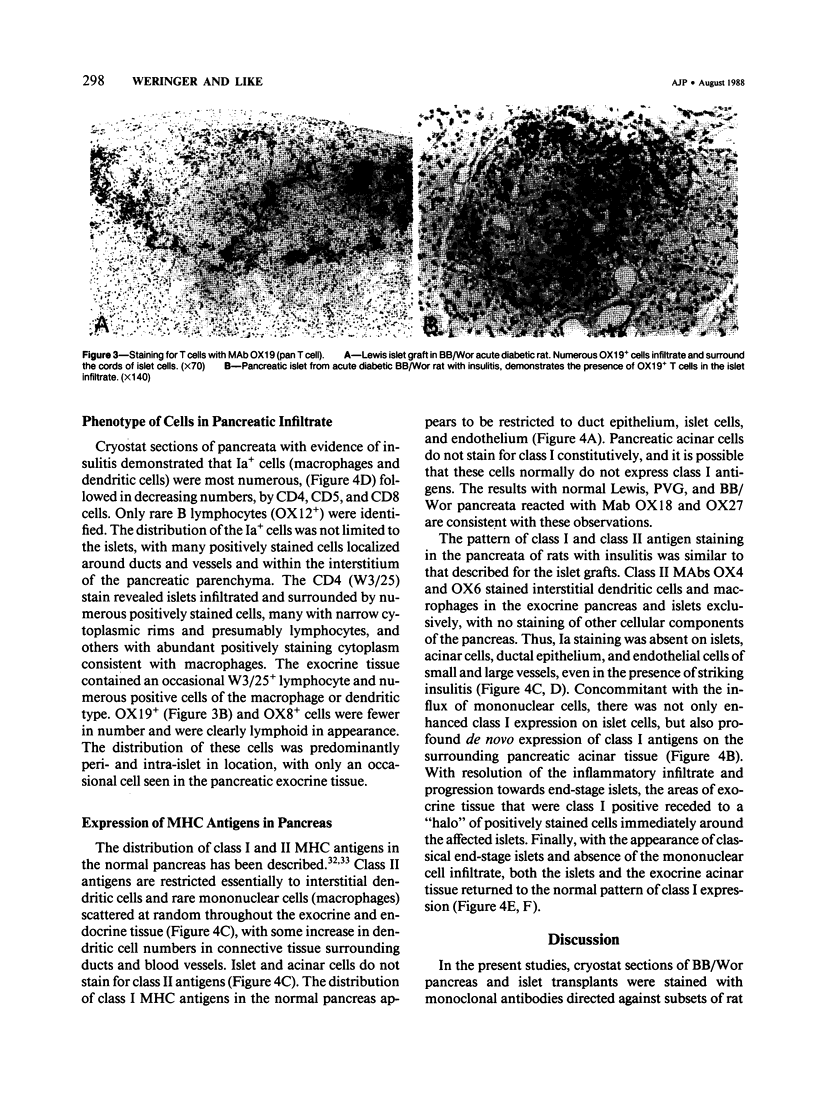
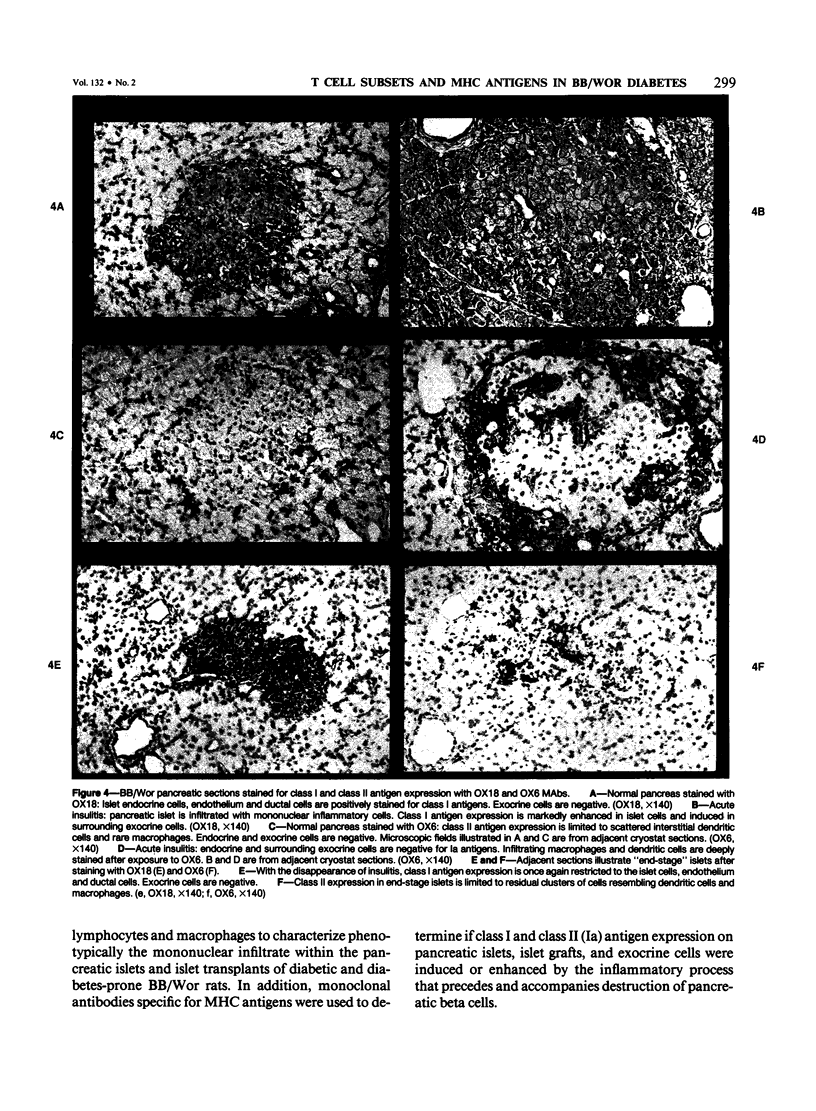




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottazzo G. F., Dean B. M., McNally J. M., MacKay E. H., Swift P. G., Gamble D. R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Robins R. A., Brooks C. G., Baldwin R. W. Phenotype of rat natural killer cells defined by monoclonal antibodies marking rat lymphocyte subsets. Immunology. 1982 Jan;45(1):97–103. [PMC free article] [PubMed] [Google Scholar]
- Colle E., Guttmann R. D., Seemayer T. Spontaneous diabetes mellitus syndrome in the rat. I. Association with the major histocompatibility complex. J Exp Med. 1981 Oct 1;154(4):1237–1242. doi: 10.1084/jem.154.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman M. J., Mason D. W., Webb M. The roles of host and donor cells in the rejection of skin allografts by T cell-deprived rats injected with syngeneic T cells. Eur J Immunol. 1982 Jun;12(6):511–518. doi: 10.1002/eji.1830120612. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Thomas M. L., Green J. R. MRC OX-19: a monoclonal antibody that labels rat T lymphocytes and augments in vitro proliferative responses. Eur J Immunol. 1984 Mar;14(3):260–267. doi: 10.1002/eji.1830140311. [DOI] [PubMed] [Google Scholar]
- Dean B. M., Walker R., Bone A. J., Baird J. D., Cooke A. Pre-diabetes in the spontaneously diabetic BB/E rat: lymphocyte subpopulations in the pancreatic infiltrate and expression of rat MHC class II molecules in endocrine cells. Diabetologia. 1985 Jul;28(7):464–466. doi: 10.1007/BF00280892. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Dyrberg T., Nakhooda A. F., Baekkeskov S., Lernmark A., Poussier P., Marliss E. B. Islet cell surface antibodies and lymphocyte antibodies in the spontaneously diabetic BB Wistar rat. Diabetes. 1982 Mar;31(3):278–281. doi: 10.2337/diab.31.3.278. [DOI] [PubMed] [Google Scholar]
- Elder M., Maclaren N., Riley W., McConnell T. Gastric parietal cell and other autoantibodies in the BB rat. Diabetes. 1982 Apr;31(4 Pt 1):313–318. doi: 10.2337/diab.31.4.313. [DOI] [PubMed] [Google Scholar]
- Foulis A. K., Farquharson M. A., Hardman R. Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987 May;30(5):333–343. doi: 10.1007/BF00299027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto T., McMaster W. R., Williams A. F. Mouse monoclonal antibodies against rat major histocompatibility antigens. Two Ia antigens and expression of Ia and class I antigens in rat thymus. Eur J Immunol. 1982 Mar;12(3):237–243. doi: 10.1002/eji.1830120313. [DOI] [PubMed] [Google Scholar]
- Green J., Jotte R. Interactions between T helper cells and dendritic cells during the rat mixed lymphocyte reaction. J Exp Med. 1985 Nov 1;162(5):1546–1560. doi: 10.1084/jem.162.5.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981 Aug 1;154(2):347–361. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Endogenously produced Ia antigens within cells of convoluted tubules of rat kidney. J Immunol. 1981 Jun;126(6):2109–2113. [PubMed] [Google Scholar]
- Hart D. N., Newton M. R., Reece-Smith H., Fabre J. W., Morris P. J. Major histocompatibility complex antigens in the rat pancreas, isolated pancreatic islets, thyroid, and adrenal. Transplantation. 1983 Oct;36(4):431–435. doi: 10.1097/00007890-198310000-00015. [DOI] [PubMed] [Google Scholar]
- Hunt S. V., Fowler M. H. A repopulation assay for B and T lymphocyte stem cells employing radiation chimaeras. Cell Tissue Kinet. 1981 Jul;14(4):445–464. doi: 10.1111/j.1365-2184.1981.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Issa-Chergui B., Yale J. F., Vigeant C., Seemayer T. A. Major histocompatibility complex gene product expression on pancreatic beta cells in acutely diabetic BB rats. Am J Pathol. 1988 Jan;130(1):156–162. [PMC free article] [PubMed] [Google Scholar]
- Jackson R. A., Buse J. B., Rifai R., Pelletier D., Milford E. L., Carpenter C. B., Eisenbarth G. S., Williams R. M. Two genes required for diabetes in BB rats. Evidence from cyclical intercrosses and backcrosses. J Exp Med. 1984 Jun 1;159(6):1629–1636. doi: 10.1084/jem.159.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koevary S. B., Williams D. E., Williams R. M., Chick W. L. Passive transfer of diabetes from BB/W to Wistar-Furth rats. J Clin Invest. 1985 Jun;75(6):1904–1907. doi: 10.1172/JCI111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koevary S., Rossini A., Stoller W., Chick W., Williams R. M. Passive transfer of diabetes in the BB/W rat. Science. 1983 May 13;220(4598):727–728. doi: 10.1126/science.6836309. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lafferty K. J., Bootes A., Dart G., Talmage D. W. Effect of organ culture on the survival of thyroid allografts in mice. Transplantation. 1976 Aug;22(2):138–149. doi: 10.1097/00007890-197608000-00009. [DOI] [PubMed] [Google Scholar]
- Laupacis A., Stiller C. R., Gardell C., Keown P., Dupre J., Wallace A. C., Thibert P. Cyclosporin prevents diabetes in BB Wistar rats. Lancet. 1983 Jan 1;1(8314-5):10–12. doi: 10.1016/s0140-6736(83)91558-1. [DOI] [PubMed] [Google Scholar]
- Like A. A., Anthony M., Guberski D. L., Rossini A. A. Spontaneous diabetes mellitus in the BB/W rat. Effects of glucocorticoids, cyclosporin-A, and antiserum to rat lymphocytes. Diabetes. 1983 Apr;32(4):326–330. doi: 10.2337/diab.32.4.326. [DOI] [PubMed] [Google Scholar]
- Like A. A., Appel M. C., Rossini A. A. Autoantibodies in the BB/W rat. Diabetes. 1982 Sep;31(9):816–820. doi: 10.2337/diab.31.9.816. [DOI] [PubMed] [Google Scholar]
- Like A. A., Biron C. A., Weringer E. J., Byman K., Sroczynski E., Guberski D. L. Prevention of diabetes in BioBreeding/Worcester rats with monoclonal antibodies that recognize T lymphocytes or natural killer cells. J Exp Med. 1986 Oct 1;164(4):1145–1159. doi: 10.1084/jem.164.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Like A. A., Dirodi V., Thomas S., Guberski D. L., Rossini A. A. Prevention of diabetes mellitus in the BB/W rat with Cyclosporin-A. Am J Pathol. 1984 Oct;117(1):92–97. [PMC free article] [PubMed] [Google Scholar]
- Like A. A., Kislauskis E., Williams R. R., Rossini A. A. Neonatal thymectomy prevents spontaneous diabetes mellitus in the BB/W rat. Science. 1982 May 7;216(4546):644–646. doi: 10.1126/science.7041259. [DOI] [PubMed] [Google Scholar]
- Like A. A., Rossini A. A., Guberski D. L., Appel M. C., Williams R. M. Spontaneous diabetes mellitus: reversal and prevention in the BB/W rat with antiserum to rat lymphocytes. Science. 1979 Dec 21;206(4425):1421–1423. doi: 10.1126/science.388619. [DOI] [PubMed] [Google Scholar]
- Like A. A., Weringer E. J., Holdash A., McGill P., Atkinson D., Rossini A. A. Adoptive transfer of autoimmune diabetes mellitus in biobreeding/Worcester (BB/W) inbred and hybrid rats. J Immunol. 1985 Mar;134(3):1583–1587. [PubMed] [Google Scholar]
- Logothetopoulos J., Valiquette N., Madura E., Cvet D. The onset and progression of pancreatic insulitis in the overt, spontaneously diabetic, young adult BB rat studied by pancreatic biopsy. Diabetes. 1984 Jan;33(1):33–36. doi: 10.2337/diab.33.1.33. [DOI] [PubMed] [Google Scholar]
- MacKay P., Jacobson J., Rabinovitch A. Spontaneous diabetes mellitus in the Bio-Breeding/Worcester rat. Evidence in vitro for natural killer cell lysis of islet cells. J Clin Invest. 1986 Mar;77(3):916–924. doi: 10.1172/JCI112390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Dallman M. J., Arthur R. P., Morris P. J. Mechanisms of allograft rejection: the roles of cytotoxic T-cells and delayed-type hypersensitivity. Immunol Rev. 1984;77:167–184. doi: 10.1111/j.1600-065x.1984.tb00721.x. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Naji A., Silvers W. K., Bellgrau D., Barker C. F. Spontaneous diabetes in rats: destruction of islets is prevented by immunological tolerance. Science. 1981 Sep 18;213(4514):1390–1392. doi: 10.1126/science.6791286. [DOI] [PubMed] [Google Scholar]
- Nakhooda A. F., Like A. A., Chappel C. I., Murray F. T., Marliss E. B. The spontaneously diabetic Wistar rat. Metabolic and morphologic studies. Diabetes. 1977 Feb;26(2):100–112. doi: 10.2337/diab.26.2.100. [DOI] [PubMed] [Google Scholar]
- Pipelleers D. G., in 't Veld P. A., Pipeleers-Marichal M. A., Gepts W., van de Winkel M. Presence of pancreatic hormones in islet cells with MHC-class II antigen expression. Diabetes. 1987 Jul;36(7):872–876. doi: 10.2337/diab.36.7.872. [DOI] [PubMed] [Google Scholar]
- Prud'homme G. J., Fuks A., Guttmann R. D., Colle E. T cell hybrids with specificity for islet cell antigens. J Immunol. 1986 Mar 1;136(5):1535–1536. [PubMed] [Google Scholar]
- Pujol-Borrell R., Todd I., Doshi M., Bottazzo G. F., Sutton R., Gray D., Adolf G. R., Feldmann M. HLA class II induction in human islet cells by interferon-gamma plus tumour necrosis factor or lymphotoxin. Nature. 1987 Mar 19;326(6110):304–306. doi: 10.1038/326304a0. [DOI] [PubMed] [Google Scholar]
- Robinson A. P., Puklavec M., Mason D. W. MRC OX-52: a rat T-cell antigen. Immunology. 1986 Apr;57(4):527–531. [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini A. A., Faustman D., Woda B. A., Like A. A., Szymanski I., Mordes J. P. Lymphocyte transfusions prevent diabetes in the Bio-Breeding/Worcester rat. J Clin Invest. 1984 Jul;74(1):39–46. doi: 10.1172/JCI111416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini A. A., Mordes J. P., Pelletier A. M., Like A. A. Transfusions of whole blood prevent spontaneous diabetes mellitus in the BB/W rat. Science. 1983 Feb 25;219(4587):975–977. doi: 10.1126/science.6823559. [DOI] [PubMed] [Google Scholar]
- Seemayer T. A., Tannenbaum G. S., Goldman H., Colle E. Dynamic time course studies of the spontaneously diabetic BB Wistar rat. III. Light-microscopic and ultrastructural observations of pancreatic islets of Langerhans. Am J Pathol. 1982 Feb;106(2):237–249. [PMC free article] [PubMed] [Google Scholar]
- Shih W. W., Matzinger P. C., Swain S. L., Dutton R. W. Analysis of histocompatibility requirements for proliferative and helper T cell activity. T cell populations depleted of alloreactive cells by negative selection. J Exp Med. 1980 Nov 1;152(5):1311–1328. doi: 10.1084/jem.152.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley R. K., Sutherland D. E., Goetz F., Michael A. F. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest. 1985 Aug;53(2):132–144. [PubMed] [Google Scholar]
- Sibley R. K., Sutherland D. E. Pancreas transplantation. An immunohistologic and histopathologic examination of 100 grafts. Am J Pathol. 1987 Jul;128(1):151–170. [PMC free article] [PubMed] [Google Scholar]
- Singer A., Hodes R. J. Mechanisms of T cell-B cell interaction. Annu Rev Immunol. 1983;1:211–241. doi: 10.1146/annurev.iy.01.040183.001235. [DOI] [PubMed] [Google Scholar]
- Steiniger B., Klempnauer J., Wonigeit K. Altered distribution of class I and class II MHC antigens during acute pancreas allograft rejection in the rat. Transplantation. 1985 Sep;40(3):234–239. doi: 10.1097/00007890-198509000-00002. [DOI] [PubMed] [Google Scholar]
- Steiniger B., Klempnauer J., Wonigeit K. Phenotype and histological distribution of interstitial dendritic cells in the rat pancreas, liver, heart, and kidney. Transplantation. 1984 Aug;38(2):169–174. doi: 10.1097/00007890-198408000-00016. [DOI] [PubMed] [Google Scholar]
- Sun D., Wekerle H. Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature. 1986 Mar 6;320(6057):70–72. doi: 10.1038/320070a0. [DOI] [PubMed] [Google Scholar]
- Warnke R., Levy R. Detection of T and B cell antigens hybridoma monoclonal antibodies: a biotin-avidin-horseradish peroxidase method. J Histochem Cytochem. 1980 Aug;28(8):771–776. doi: 10.1177/28.8.7003003. [DOI] [PubMed] [Google Scholar]
- Weringer E. J., Like A. A. Diabetes mellitus in the BB/W rat. Insulitis in pancreatic islet grafts after transplantation in diabetic recipients. Am J Pathol. 1986 Oct;125(1):107–112. [PMC free article] [PubMed] [Google Scholar]
- Weringer E. J., Like A. A. Immune attack on pancreatic islet transplants in the spontaneously diabetic BioBreeding/Worcester (BB/W) rat is not MHC restricted. J Immunol. 1985 Apr;134(4):2383–2386. [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Woda B. A., Like A. A., Padden C., McFadden M. L. Deficiency of phenotypic cytotoxic-suppressor T lymphocytes in the BB/W rat. J Immunol. 1986 Feb 1;136(3):856–859. [PubMed] [Google Scholar]
- Wood G. S., Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J Histochem Cytochem. 1981 Oct;29(10):1196–1204. doi: 10.1177/29.10.7028859. [DOI] [PubMed] [Google Scholar]