Abstract
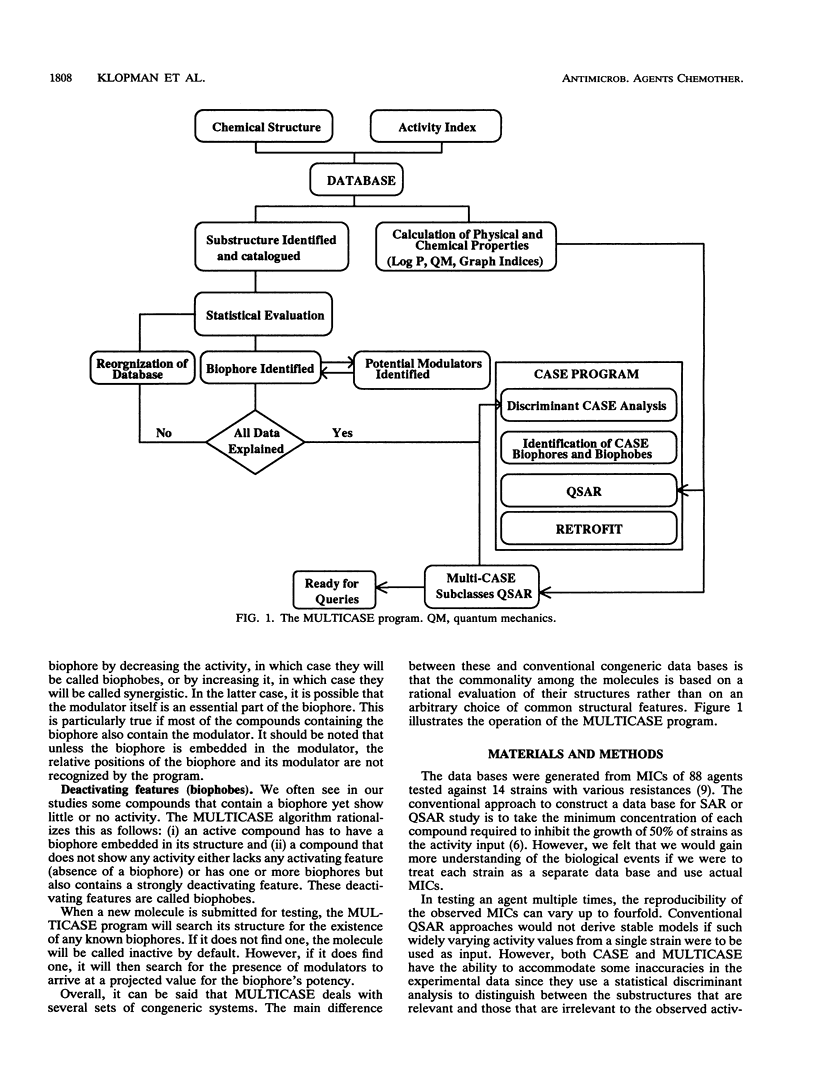
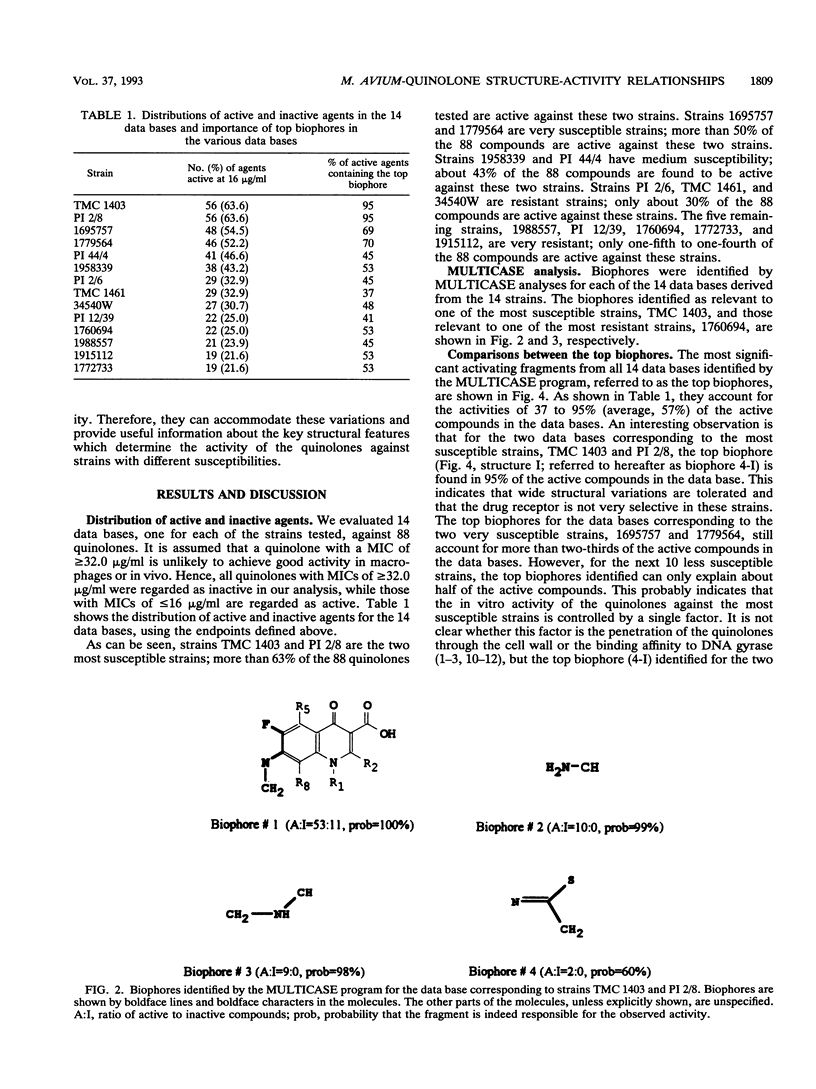
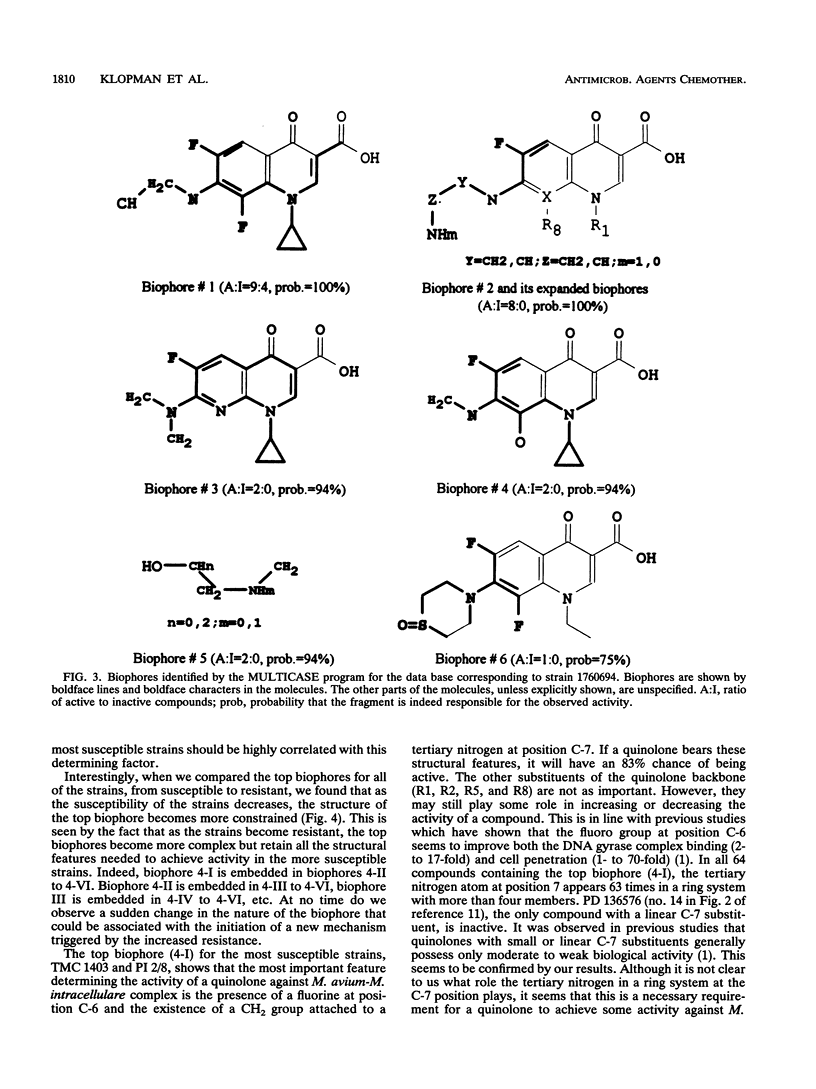
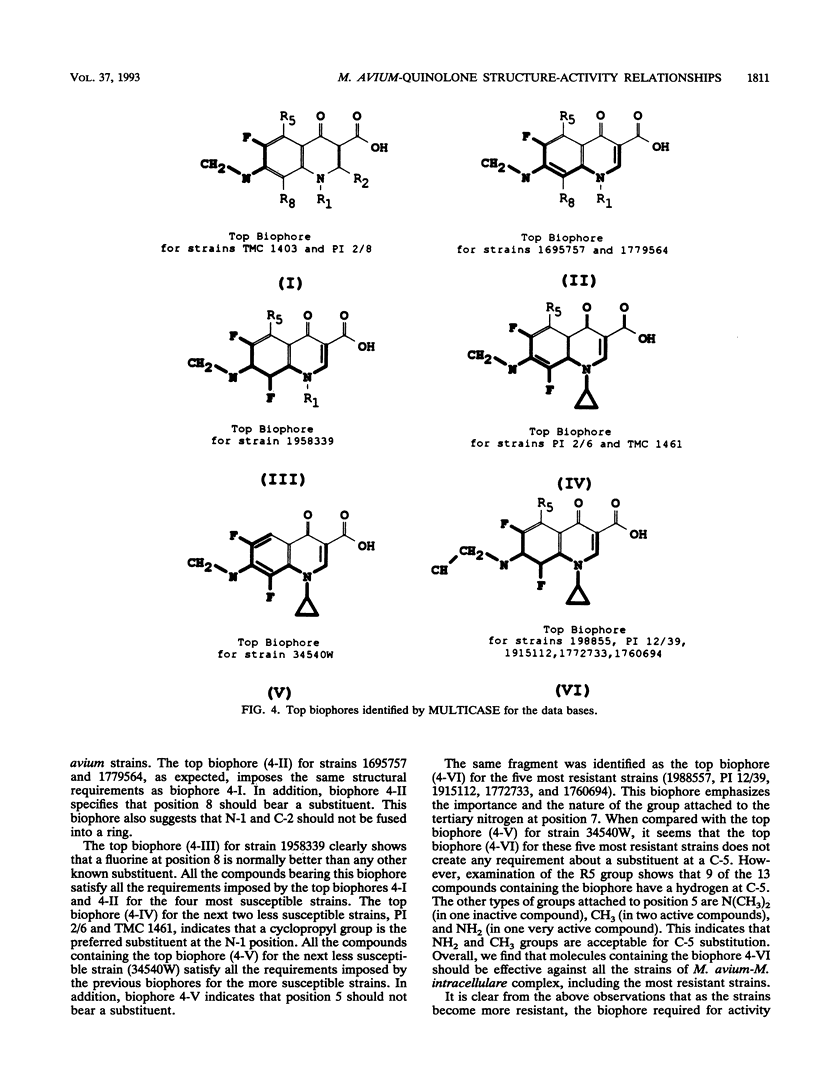
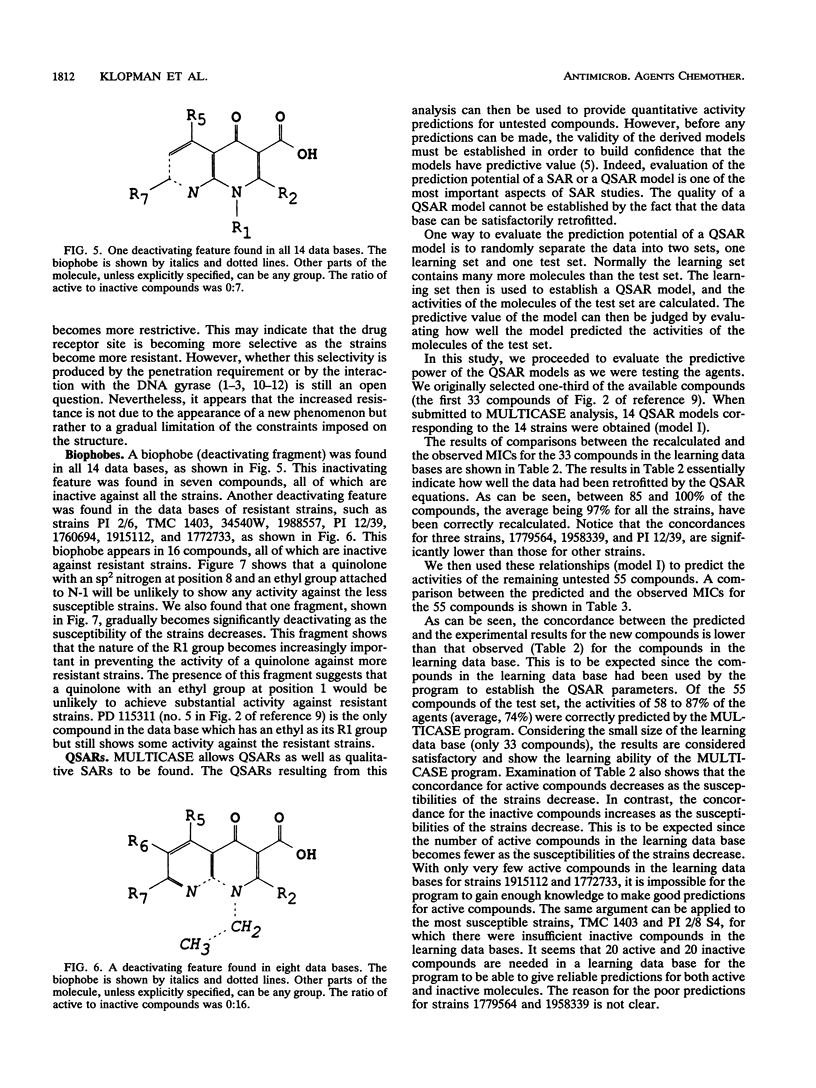
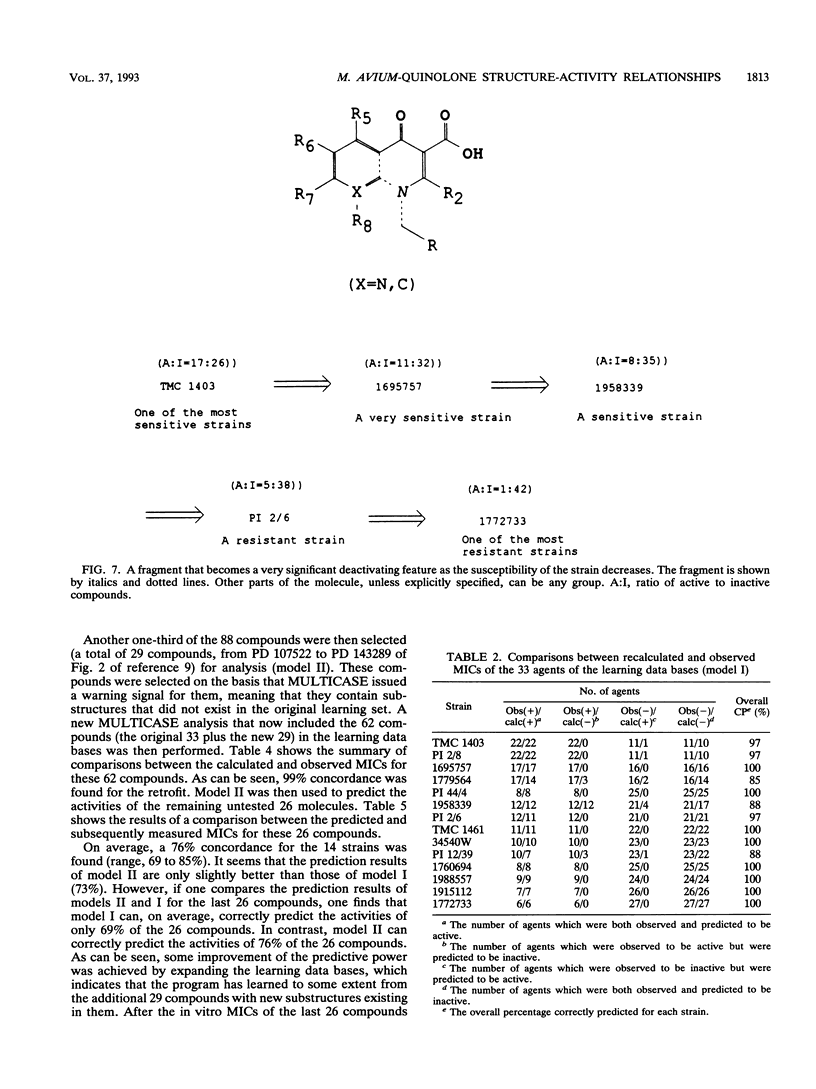
The structures and inhibitory activities of 88 quinolones, previously studied as potential in vitro inhibitors of 14 selected strains of Mycobacterium avium complex, were examined in an effort to identify a quinolone with optimal activity towards all strains. A MULTICASE structure-activity relationship analysis of the inhibitory activities of these 88 quinolones against 14 strains of M. avium was performed and led to the identification of a number of structural constraints required to overcome the resistance of most of the strains. Our data suggested that the increased resistance of the strains was probably not due to a specific resistance mechanism but rather due to gradual limitation of the constraints imposed on the structure of the quinolones. This increasing structural selectivity could be produced either at the level of cell membrane penetration or at the level of interaction with the DNA gyrase receptor site. On the basis of these findings, a number of new quinolones holding the promise of superior activity are currently being evaluated in vitro and in vivo to determine the clinical relevance of our observations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Domagala J. M., Hanna L. D., Heifetz C. L., Hutt M. P., Mich T. F., Sanchez J. P., Solomon M. New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay. J Med Chem. 1986 Mar;29(3):394–404. doi: 10.1021/jm00153a015. [DOI] [PubMed] [Google Scholar]
- Högberg T., Khanna I., Drake S. D., Mitscher L. A., Shen L. L. Structure-activity relationships among DNA gyrase inhibitors. Synthesis and biological evaluation of 1,2-dihydro-4, 4-dimethyl-1-oxo-2-naphthalenecarboxylic acids as 1-carba bioisosteres of oxolinic acid. J Med Chem. 1984 Mar;27(3):306–310. doi: 10.1021/jm00369a013. [DOI] [PubMed] [Google Scholar]
- Klopman G., Macina O. T., Levinson M. E., Rosenkranz H. S. Computer automated structure evaluation of quinolone antibacterial agents. Antimicrob Agents Chemother. 1987 Nov;31(11):1831–1840. doi: 10.1128/aac.31.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopman G., Wang S., Jacobs M. R., Bajaksouzian S., Edmonds K., Ellner J. J. Anti-Mycobacterium avium activity of quinolones: in vitro activities. Antimicrob Agents Chemother. 1993 Sep;37(9):1799–1806. doi: 10.1128/aac.37.9.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H., Itoh A., Murayama S., Suzue S., Irikura T. Structure-activity relationships of antibacterial 6,7- and 7,8-disubstituted 1-alkyl-1,4-dihydro-4-oxoquinoline-3-carboxylic acids. J Med Chem. 1980 Dec;23(12):1358–1363. doi: 10.1021/jm00186a014. [DOI] [PubMed] [Google Scholar]



