Abstract
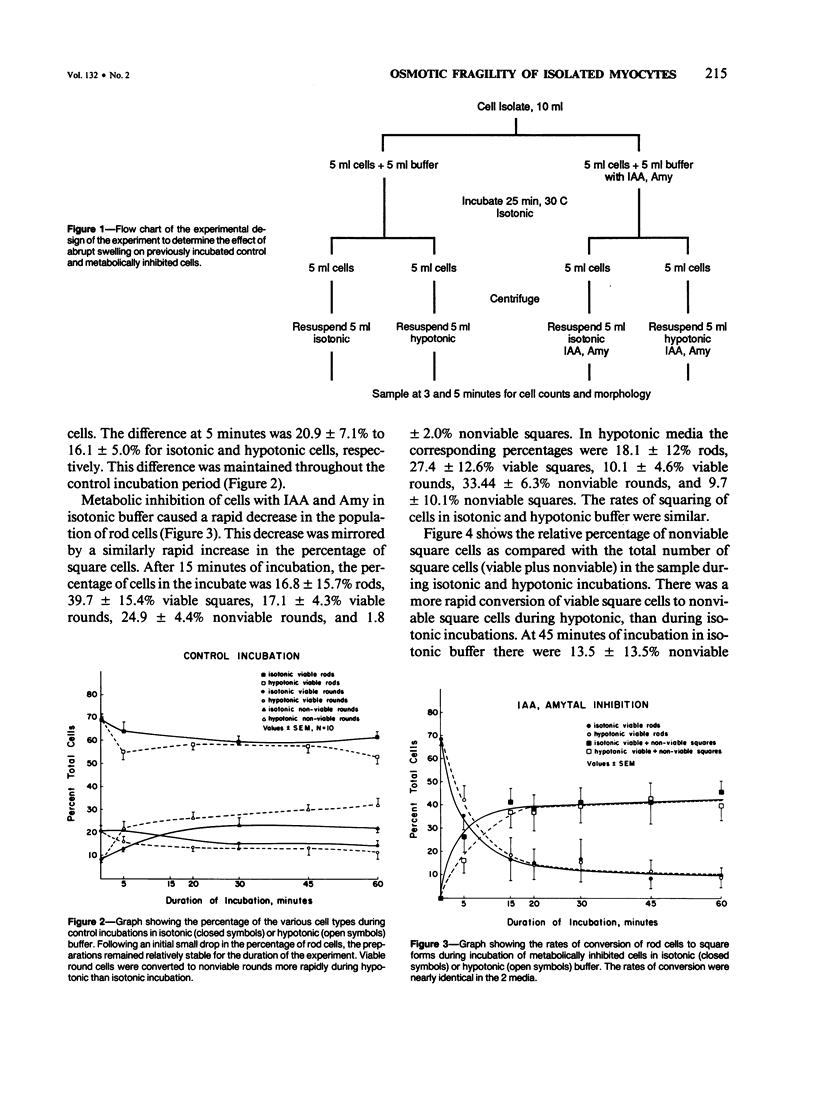
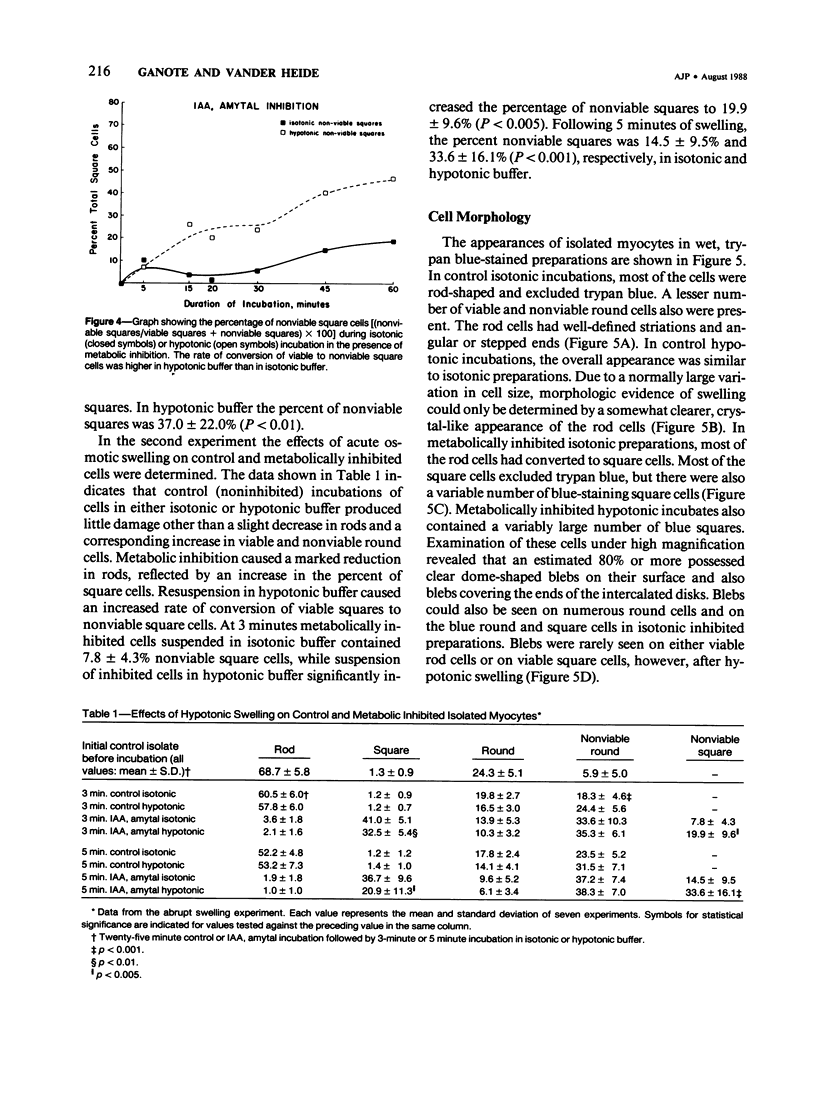
Isolated myocytes can be established as a valid model for studying changes in cytoskeletal proteins during the development of irreversible injury only if isolated cells develop lesions similar to those that occur during irreversible injury to intact hearts, specifically osmotic fragility and subsarcolemmal blebs. In the first experiment, isolated cells were irreversibly injured by metabolic inhibition with 5 mM Iodoacetic acid (IAA) and 6 mM amobarbital (Amy). Osmotic fragility of control and injured cells was determined by comparing the rates of development of trypan blue permeability during 60 minutes of isotonic or hypotonic (50% reduction in osmolality) incubations. Cell morphology was monitored by light and electron microscopy. Control cells remained elongated and excluded trypan blue. Metabolically inhibited cells rapidly contracted to a nearly square shape. The inhibited squared cells initially excluded trypan blue, but during 60 minutes of incubation became permeable to trypan blue. Cells in hypotonic buffer developed blue staining at a more rapid rate than cells in isotonic buffer, indicating increased osmotic fragility. In a second experiment, control and inhibited cells were first incubated for 25 minutes in isotonic buffer and then in either isotonic or hypotonic buffer. In this experiment, inhibited cells also developed more extensive and rapid permeability increases when transferred to the hypotonic buffer than cells maintained in the isotonic buffer. In both experiments, increased permeability of cells to trypan blue was accompanied by formation of subsarcolemmal blebs along the lateral cell border and at the intercalated disks. The results show that metabolically inhibited, isolated myocytes do exhibit morphologic lesions and increased osmotic fragility properties similar to those reported during anoxic or ischemic injury to intact hearts. Therefore, isolated myocytes may be a useful model with which to study cytoskeletal-sarcolemmal membrane changes during development of irreversible injury.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschuld R. A., Hostetler J. R., Brierley G. P. Response of isolated rat heart cells to hypoxia, re-oxygenation, and acidosis. Circ Res. 1981 Aug;49(2):307–316. doi: 10.1161/01.res.49.2.307. [DOI] [PubMed] [Google Scholar]
- Altschuld R. A., Wenger W. C., Lamka K. G., Kindig O. R., Capen C. C., Mizuhira V., Vander Heide R. S., Brierley G. P. Structural and functional properties of adult rat heart myocytes lysed with digitonin. J Biol Chem. 1985 Nov 15;260(26):14325–14334. [PubMed] [Google Scholar]
- Altschuld R., Gibb L., Ansel A., Hohl C., Kruger F. A., Brierley G. P. Calcium tolerance of isolated rat heart cells. J Mol Cell Cardiol. 1980 Dec;12(12):1383–1395. doi: 10.1016/0022-2828(80)90123-6. [DOI] [PubMed] [Google Scholar]
- Frank J. S., Brady A. J., Farnsworth S., Mottino G. Ultrastructure and function of isolated myocytes after calcium depletion and repletion. Am J Physiol. 1986 Feb;250(2 Pt 2):H265–H275. doi: 10.1152/ajpheart.1986.250.2.H265. [DOI] [PubMed] [Google Scholar]
- Ganote C. E. Contraction band necrosis and irreversible myocardial injury. J Mol Cell Cardiol. 1983 Feb;15(2):67–73. doi: 10.1016/0022-2828(83)90283-3. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Humphrey S. M. Effects of anoxic or oxygenated reperfusion in globally ischemic, isovolumic, perfused rat hearts. Am J Pathol. 1985 Jul;120(1):129–145. [PMC free article] [PubMed] [Google Scholar]
- Ganote C. E., Vander Heide R. S. Cytoskeletal lesions in anoxic myocardial injury. A conventional and high-voltage electron-microscopic and immunofluorescence study. Am J Pathol. 1987 Nov;129(2):327–344. [PMC free article] [PubMed] [Google Scholar]
- Geisbuhler T., Altschuld R. A., Trewyn R. W., Ansel A. Z., Lamka K., Brierley G. P. Adenine nucleotide metabolism and compartmentalization in isolated adult rat heart cells. Circ Res. 1984 May;54(5):536–546. doi: 10.1161/01.res.54.5.536. [DOI] [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R., Berkoff H. A. Contracture in isolated adult rat heart cells. Role of Ca2+, ATP, and compartmentation. Circ Res. 1981 Nov;49(5):1119–1128. doi: 10.1161/01.res.49.5.1119. [DOI] [PubMed] [Google Scholar]
- Herman B., Nieminen A. L., Gores G. J., Lemasters J. J. Irreversible injury in anoxic hepatocytes precipitated by an abrupt increase in plasma membrane permeability. FASEB J. 1988 Feb;2(2):146–151. doi: 10.1096/fasebj.2.2.3342967. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A., Steenbergen C. Myocardial ischemia revisited. The osmolar load, membrane damage, and reperfusion. J Mol Cell Cardiol. 1986 Aug;18(8):769–780. doi: 10.1016/s0022-2828(86)80952-x. [DOI] [PubMed] [Google Scholar]
- Lambert M. R., Johnson J. D., Lamka K. G., Brierley G. P., Altschuld R. A. Intracellular free Ca2+ and the hypercontracture of adult rat heart myocytes. Arch Biochem Biophys. 1986 Mar;245(2):426–435. doi: 10.1016/0003-9861(86)90234-1. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B. The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. I. Comparison of different perfusion fixation methods and of glutaraldehyde, formaldehyde and osmium tetroxide fixatives. J Ultrastruct Res. 1966 Jun;15(3):242–282. doi: 10.1016/s0022-5320(66)80109-0. [DOI] [PubMed] [Google Scholar]
- Mazet F., Wittenberg B. A., Spray D. C. Fate of intercellular junctions in isolated adult rat cardiac cells. Circ Res. 1985 Feb;56(2):195–204. doi: 10.1161/01.res.56.2.195. [DOI] [PubMed] [Google Scholar]
- Murphy M. P., Hohl C., Brierley G. P., Altschuld R. A. Release of enzymes from adult rat heart myocytes. Circ Res. 1982 Nov;51(5):560–568. doi: 10.1161/01.res.51.5.560. [DOI] [PubMed] [Google Scholar]
- Piper H. M., Schwartz P., Hütter J. F., Spieckermann P. G. Energy metabolism and enzyme release of cultured adult rat heart muscle cells during anoxia. J Mol Cell Cardiol. 1984 Nov;16(11):995–1007. doi: 10.1016/s0022-2828(84)80013-9. [DOI] [PubMed] [Google Scholar]
- Piper H. M., Spahr R., Hütter J. F., Spieckermann P. G. The calcium and the oxygen paradox: non-existent on the cellular level. Basic Res Cardiol. 1985;80 (Suppl 2):159–163. [PubMed] [Google Scholar]
- Pontremoli S., Melloni E. Extralysosomal protein degradation. Annu Rev Biochem. 1986;55:455–481. doi: 10.1146/annurev.bi.55.070186.002323. [DOI] [PubMed] [Google Scholar]
- Reddy M. K., Rabinowitz M., Zak R. Stringent requirement for Ca2+ in the removal of Z-lines and alpha-actinin from isolated myofibrils by Ca2+-activated neutral proteinase. Biochem J. 1983 Mar 1;209(3):635–641. doi: 10.1042/bj2090635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz P., Piper H. M., Spahr R., Spieckermann P. G. Ultrastructure of cultured adult myocardial cells during anoxia and reoxygenation. Am J Pathol. 1984 Jun;115(3):349–361. [PMC free article] [PubMed] [Google Scholar]
- Steenbergen C., Hill M. L., Jennings R. B. Cytoskeletal damage during myocardial ischemia: changes in vinculin immunofluorescence staining during total in vitro ischemia in canine heart. Circ Res. 1987 Apr;60(4):478–486. doi: 10.1161/01.res.60.4.478. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Hill M. L., Jennings R. B. Volume regulation and plasma membrane injury in aerobic, anaerobic, and ischemic myocardium in vitro. Effects of osmotic cell swelling on plasma membrane integrity. Circ Res. 1985 Dec;57(6):864–875. doi: 10.1161/01.res.57.6.864. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Chien A. M., Capogrossi M. C., Pelto D. J., Lakatta E. G. Direct observation of the "oxygen paradox" in single rat ventricular myocytes. Circ Res. 1985 Jun;56(6):899–903. doi: 10.1161/01.res.56.6.899. [DOI] [PubMed] [Google Scholar]
- Vander Heide R. S., Angelo J. P., Altschuld R. A., Ganote C. E. Energy dependence of contraction band formation in perfused hearts and isolated adult myocytes. Am J Pathol. 1986 Oct;125(1):55–68. [PMC free article] [PubMed] [Google Scholar]
- Vander Heide R. S., Ganote C. E. Increased myocyte fragility following anoxic injury. J Mol Cell Cardiol. 1987 Nov;19(11):1085–1103. doi: 10.1016/s0022-2828(87)80353-x. [DOI] [PubMed] [Google Scholar]
- Wenger W. C., Murphy M. P., Kindig O. R., Capen C. C., Brierley G. P., Altschuld R. A. Mitochondrial enzyme retention by irreversibly damaged rectangular isolated adult rat heart myocytes. Life Sci. 1985 Nov 4;37(18):1697–1704. doi: 10.1016/0024-3205(85)90297-8. [DOI] [PubMed] [Google Scholar]






