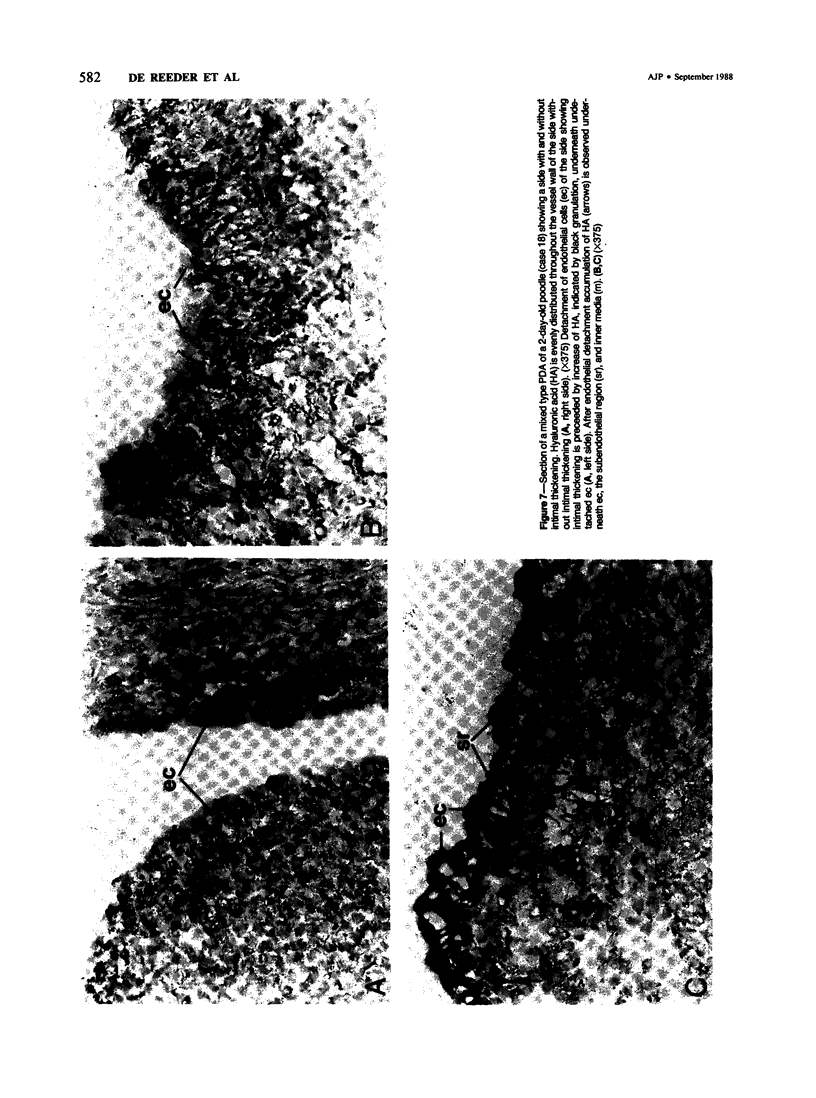
Abstract
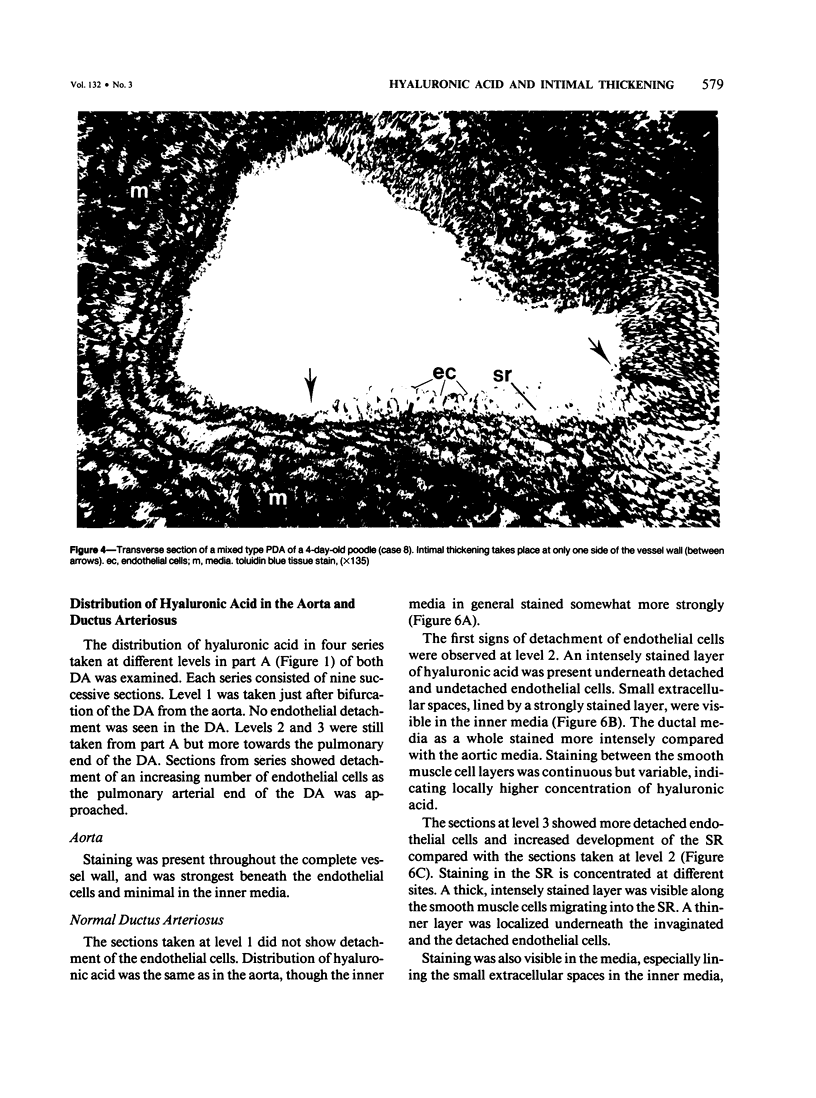
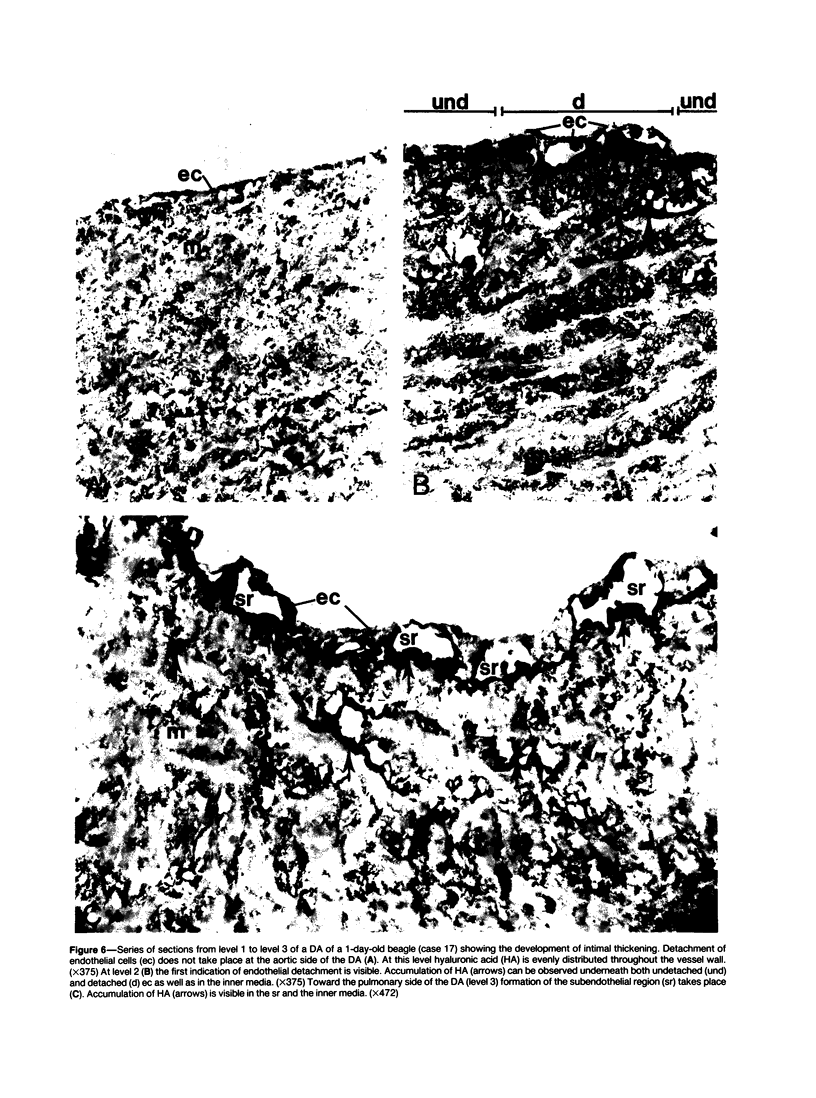
The closing ductus arteriosus (DA) was studied as a model for the development of intimal thickening of vessel walls using ultrastructural and immunohistochemical techniques. The material consisted of DA from neonatal dogs of three types: normal beagles, DA-defective pups from a line of mixed poodles with a genetic defect in the closure of the DA leading to persistent ductus arteriosus (PDA line), and normal litter-mates of DA-defective pups in the PDA line. The DA of the normal litter-mates of DA-defective pups did not differ from those of normal beagles. In the DA of normal beagles and normal PDA-line pups, closure is preceded by intimal thickening characterized by formation of a widened subendothelial region (SR), detachment of endothelial cells, invagination of endothelial cells, and migration of smooth muscle cells into the SR. It was observed that immediately before and after endothelial cell detachment, there was an increase in hyaluronic acid (HA) in the SR and inner media. In the DA-defective pups, the increase in hyaluronic acid failed to occur and there was no intimal thickening. The SR failed to expand, endothelium remained attached to the internal elastic membrane, and there was no invagination of endothelium or migration of smooth muscle cells. It is hypothesized that the increased synthesis of HA is an important early event leading to intimal thickening in the normal DA and perhaps to abnormal intimal thickening of other vessels. By its hygroscopic properties, HA may be directly involved in the formation of a wide SR, inducing endothelial cell detachment and favoring smooth muscle cell migration. In affected pups of the PDA line, there is a genetically-determined "block" in the normal process of intimal thickening at or before the initiation of increased HA synthesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausprunk D. H., Boudreau C. L., Nelson D. A. Proteoglycans in the microvascular. II. Histochemical localization in proliferating capillaries of the rabbit cornea. Am J Pathol. 1981 Jun;103(3):367–375. [PMC free article] [PubMed] [Google Scholar]
- Bartholomew J. S., Anderson J. C. Distribution of proteoglycans and hyaluronic acid in transverse sections of bovine thoracic aorta. Histochem J. 1983 Oct;15(10):941–951. doi: 10.1007/BF01002490. [DOI] [PubMed] [Google Scholar]
- Burgdorf W. H., Mukai K., Rosai J. Immunohistochemical identification of factor VIII-related antigen in endothelial cells of cutaneous lesions of alleged vascular nature. Am J Clin Pathol. 1981 Feb;75(2):167–171. doi: 10.1093/ajcp/75.2.167. [DOI] [PubMed] [Google Scholar]
- Delpech B. Immunochemical characterization of the hyaluronic acid-hyaluronectin interaction. J Neurochem. 1982 Apr;38(4):978–984. doi: 10.1111/j.1471-4159.1982.tb05338.x. [DOI] [PubMed] [Google Scholar]
- Feinberg R. N., Beebe D. C. Hyaluronate in vasculogenesis. Science. 1983 Jun 10;220(4602):1177–1179. doi: 10.1126/science.6857242. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Elemer G., Guelpa C., Vallotton M. B., Badonnel M. C., Hüttner I. Morphologic and functional changes of the aortic intima during experimental hypertension. Am J Pathol. 1979 Aug;96(2):399–422. [PMC free article] [PubMed] [Google Scholar]
- Girard N., Delpech A., Delpech B. Characterization of hyaluronic acid on tissue sections with hyaluronectin. J Histochem Cytochem. 1986 Apr;34(4):539–541. doi: 10.1177/34.4.2419397. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot A. C., Strengers J. L., Mentink M., Poelmann R. E., Patterson D. F. Histologic studies on normal and persistent ductus arteriosus in the dog. J Am Coll Cardiol. 1985 Aug;6(2):394–404. doi: 10.1016/s0735-1097(85)80178-9. [DOI] [PubMed] [Google Scholar]
- Kowala M. C., Cuénoud H. F., Joris I., Majno G. Cellular changes during hypertension: a quantitative study of the rat aorta. Exp Mol Pathol. 1986 Dec;45(3):323–335. doi: 10.1016/0014-4800(86)90021-3. [DOI] [PubMed] [Google Scholar]
- Kujawa M. J., Pechak D. G., Fiszman M. Y., Caplan A. I. Hyaluronic acid bonded to cell culture surfaces inhibits the program of myogenesis. Dev Biol. 1986 Jan;113(1):10–16. doi: 10.1016/0012-1606(86)90103-x. [DOI] [PubMed] [Google Scholar]
- Mukai K., Rosai J., Burgdorf W. H. Localization of factor VIII-related antigen in vascular endothelial cells using an immunoperoxidase method. Am J Surg Pathol. 1980 Jun;4(3):273–276. doi: 10.1097/00000478-198006000-00008. [DOI] [PubMed] [Google Scholar]
- Murata K., Nakazawa K., Hamai A. Distribution of acidic glycosaminoglycans in the intima, media and adventitia of bovine aorta and their anticoagulant properties. Atherosclerosis. 1975 Jan-Feb;21(1):93–103. doi: 10.1016/0021-9150(75)90096-9. [DOI] [PubMed] [Google Scholar]
- Ordóez N. G., Batsakis J. G. Comparison of Ulex europaeus I lectin and factor VIII-related antigen in vascular lesions. Arch Pathol Lab Med. 1984 Feb;108(2):129–132. [PubMed] [Google Scholar]
- Owens G. K., Reidy M. A. Hyperplastic growth response of vascular smooth muscle cells following induction of acute hypertension in rats by aortic coarctation. Circ Res. 1985 Nov;57(5):695–705. doi: 10.1161/01.res.57.5.695. [DOI] [PubMed] [Google Scholar]
- Patterson D. F. Epidemiologic and genetic studies of congenital heart disease in the dog. Circ Res. 1968 Aug;23(2):171–202. doi: 10.1161/01.res.23.2.171. [DOI] [PubMed] [Google Scholar]
- Patterson D. F., Pyle R. L., Buchanan J. W., Trautvetter E., Abt D. A. Hereditary patent ductus arteriosus and its sequelae in the dog. Circ Res. 1971 Jul;29(1):1–13. doi: 10.1161/01.res.29.1.1. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Salisbury B. G., Wagner W. D. Isolation and preliminary characterization of proteoglycans dissociatively extracted from human aorta. J Biol Chem. 1981 Aug 10;256(15):8050–8057. [PubMed] [Google Scholar]
- Schlingemann R. O., Dingjan G. M., Emeis J. J., Blok J., Warnaar S. O., Ruiter D. J. Monoclonal antibody PAL-E specific for endothelium. Lab Invest. 1985 Jan;52(1):71–76. [PubMed] [Google Scholar]
- Scott L. J., Merrilees M. J. Stimulation of smooth muscle cell glycosaminoglycan synthesis by cultured endothelial cells is dependent on endothelial cell density. Atherosclerosis. 1987 Feb;63(2-3):145–152. doi: 10.1016/0021-9150(87)90114-6. [DOI] [PubMed] [Google Scholar]
- Stel H. V., Sakariassen K. S., Scholte B. J., Veerman E. C., van der Kwast T. H., de Groot P. G., Sixma J. J., van Mourik J. A. Characterization of 25 monoclonal antibodies to factor VIII-von Willebrand factor: relationship between ristocetin-induced platelet aggregation and platelet adherence to subendothelium. Blood. 1984 Jun;63(6):1408–1415. [PubMed] [Google Scholar]
- Still W. J. The early effect of hypertension on the aortic intima of the rat. An electron microscopic study. Am J Pathol. 1967 Nov;51(5):721–734. [PMC free article] [PubMed] [Google Scholar]
- Toole B. P. Developmental role of hyaluronate. Connect Tissue Res. 1982;10(1):93–100. doi: 10.3109/03008208209034409. [DOI] [PubMed] [Google Scholar]
- Van Hoof J., Harrisson F., Andries L., Vakaet L. Microinjection of glycosaminoglycan-degrading enzymes in the chicken blastoderm. An ultrastructural study. Differentiation. 1986;31(1):14–19. doi: 10.1111/j.1432-0436.1986.tb00377.x. [DOI] [PubMed] [Google Scholar]
- West D. C., Hampson I. N., Arnold F., Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985 Jun 14;228(4705):1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Nikkari T., Hirvonen J., Laaksonen H., Möttönen M., Pesonen E., Raekallio J., Akerblom H. K. Biochemical composition of coronary arteries in Finnish children. Arteriosclerosis. 1986 Mar-Apr;6(2):230–236. doi: 10.1161/01.atv.6.2.230. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Sumuvuori H., Karkola K., Möttönen M., Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest. 1986 Apr;54(4):402–407. [PubMed] [Google Scholar]