Abstract
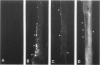
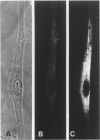
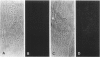
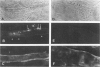
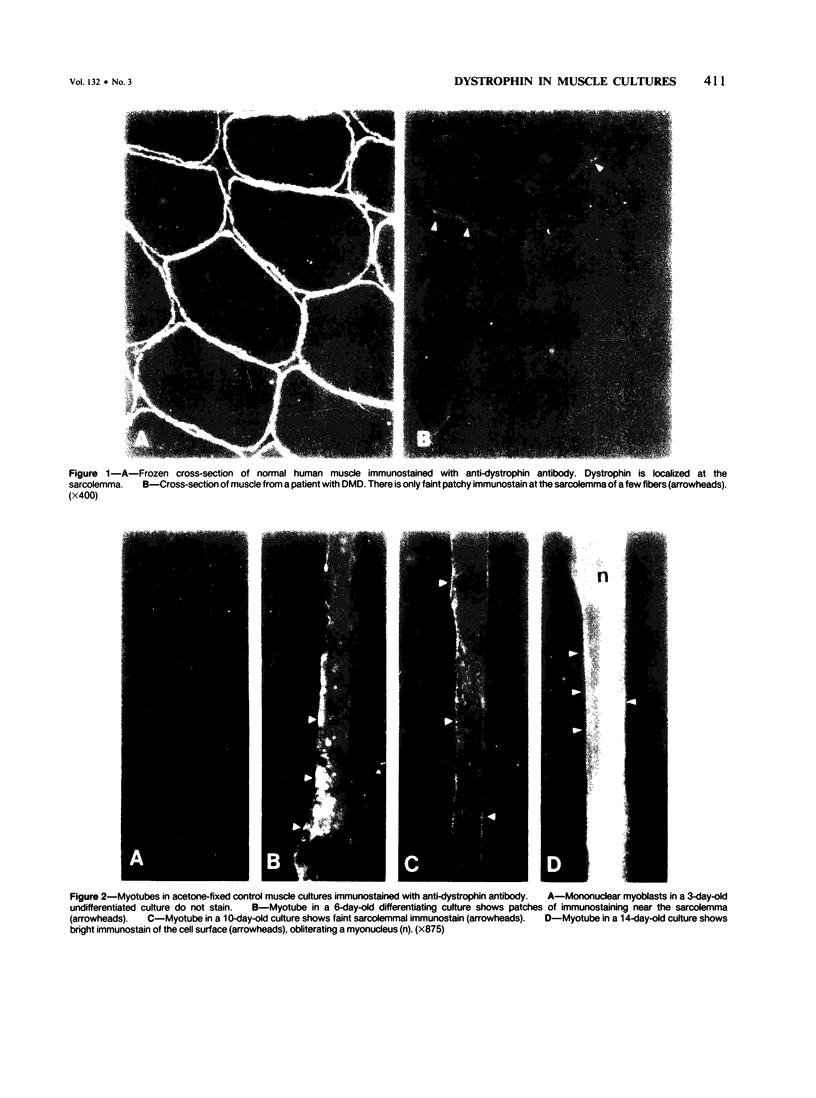
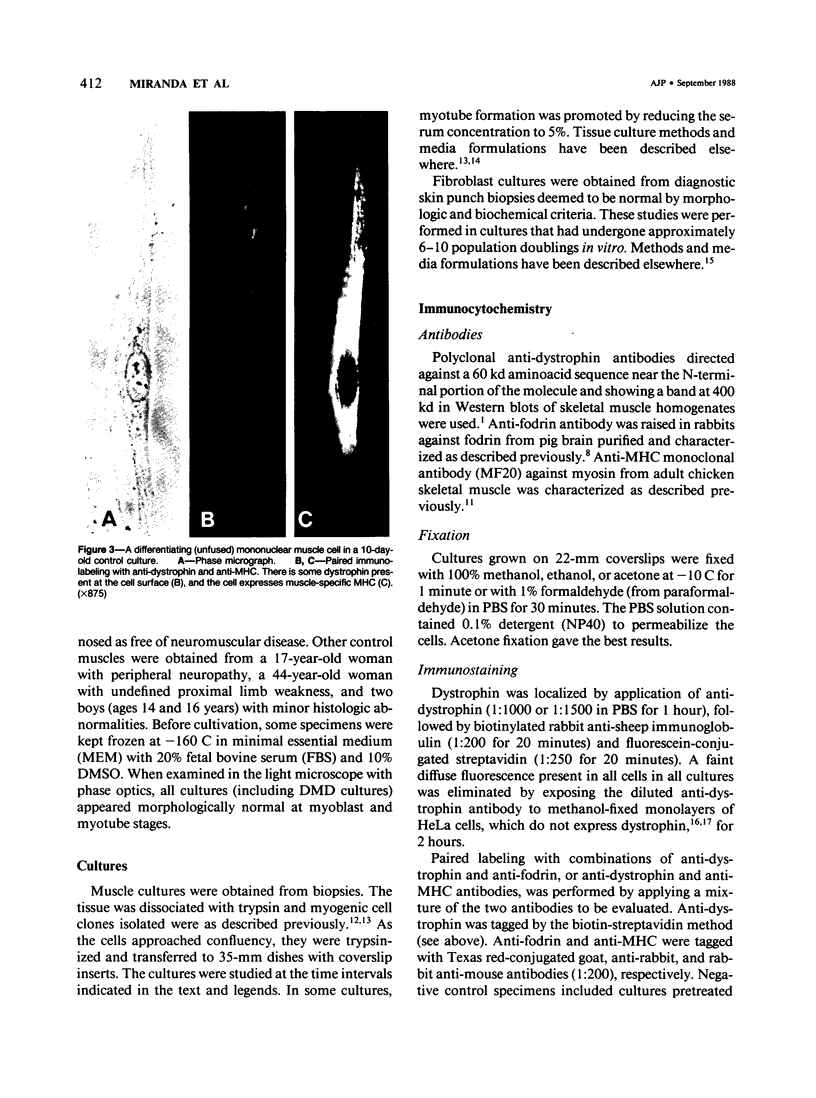
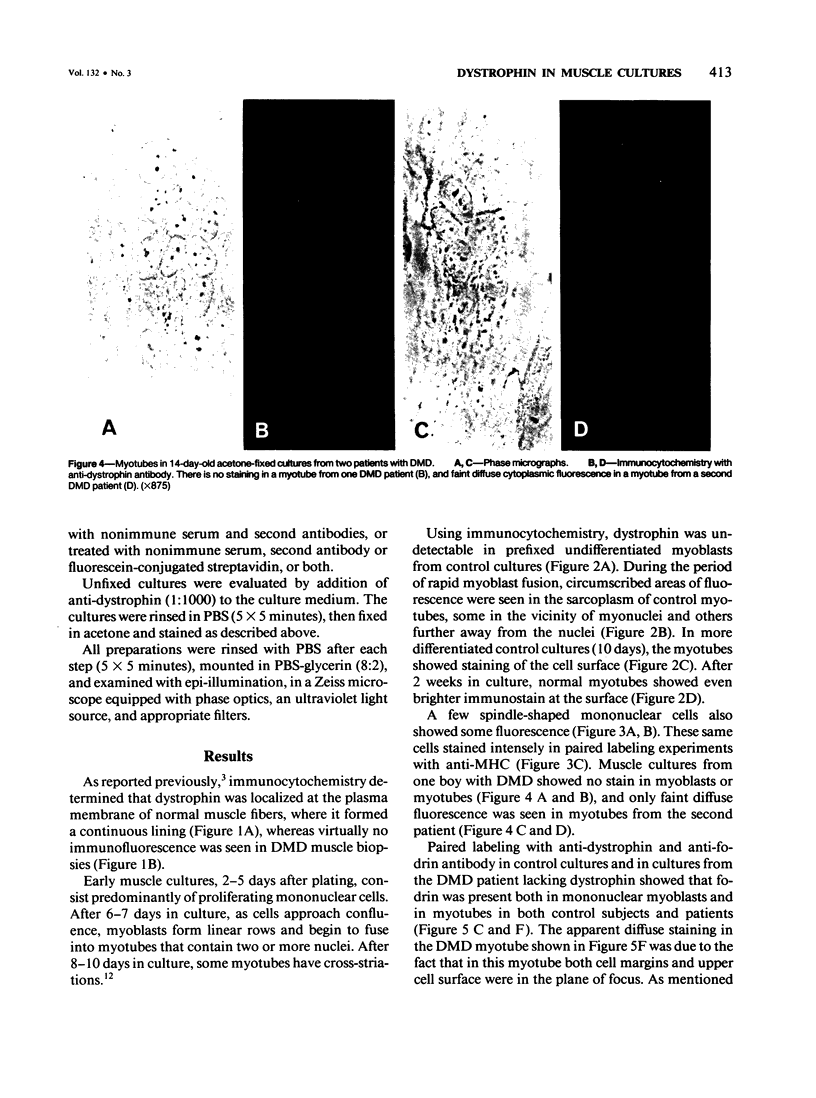
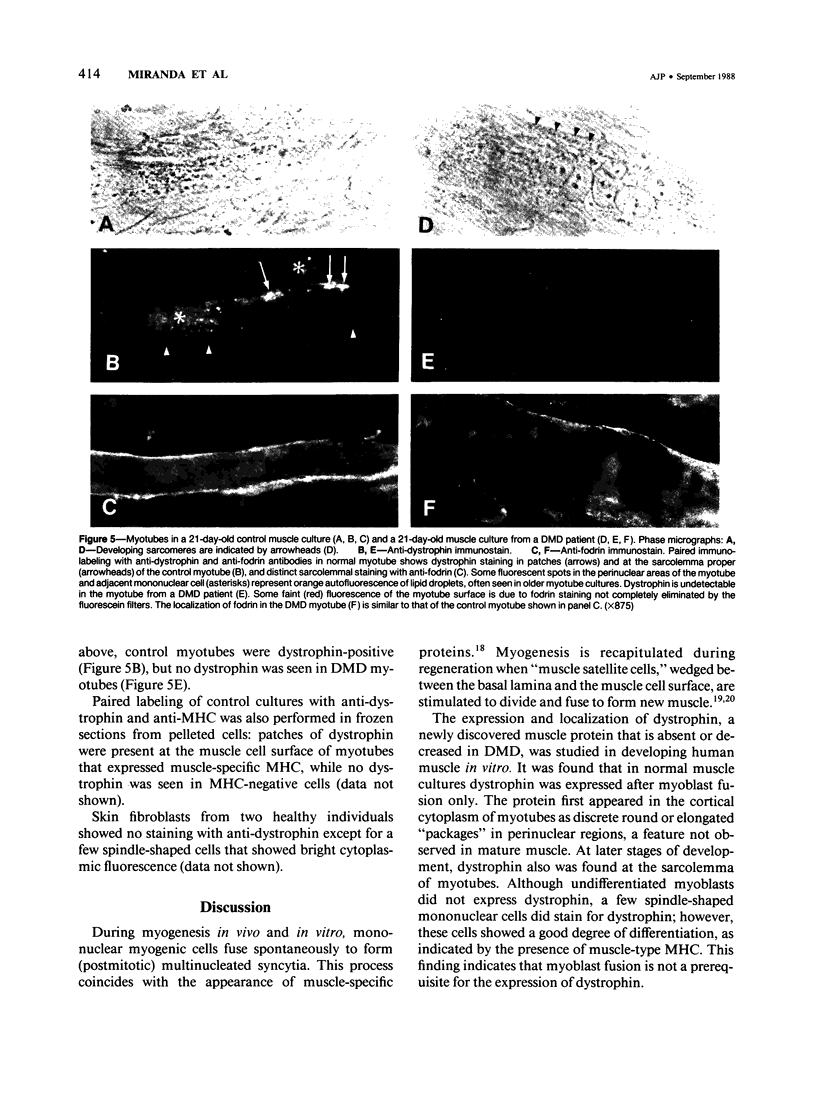
Using immunocytochemical methods, the localization of dystrophin, the gene product affected in Duchenne muscular dystrophy (DMD) in aneural, differentiating human muscle cultures, was studied. Dystrophin was not demonstrable in undifferentiated myoblasts from control patients and from two patients with DMD. After myoblast fusion, the protein was found in circumscribed sarcoplasmic patches, in the perinuclear area, and along the surface of all normal multinucleate myotubes, with more mature myotubes showing predominantly sarcolemmal distribution. There was no staining in myotubes from one DMD patient and only faint diffuse fluorescence in myotubes from the second affected boy, however. These data provide further evidence that dystrophin is a sarcolemma-associated protein, that it is developmentally regulated, and that it is absent or greatly reduced in quantity in skeletal muscle cultures from patients with DMD.
Full text
PDF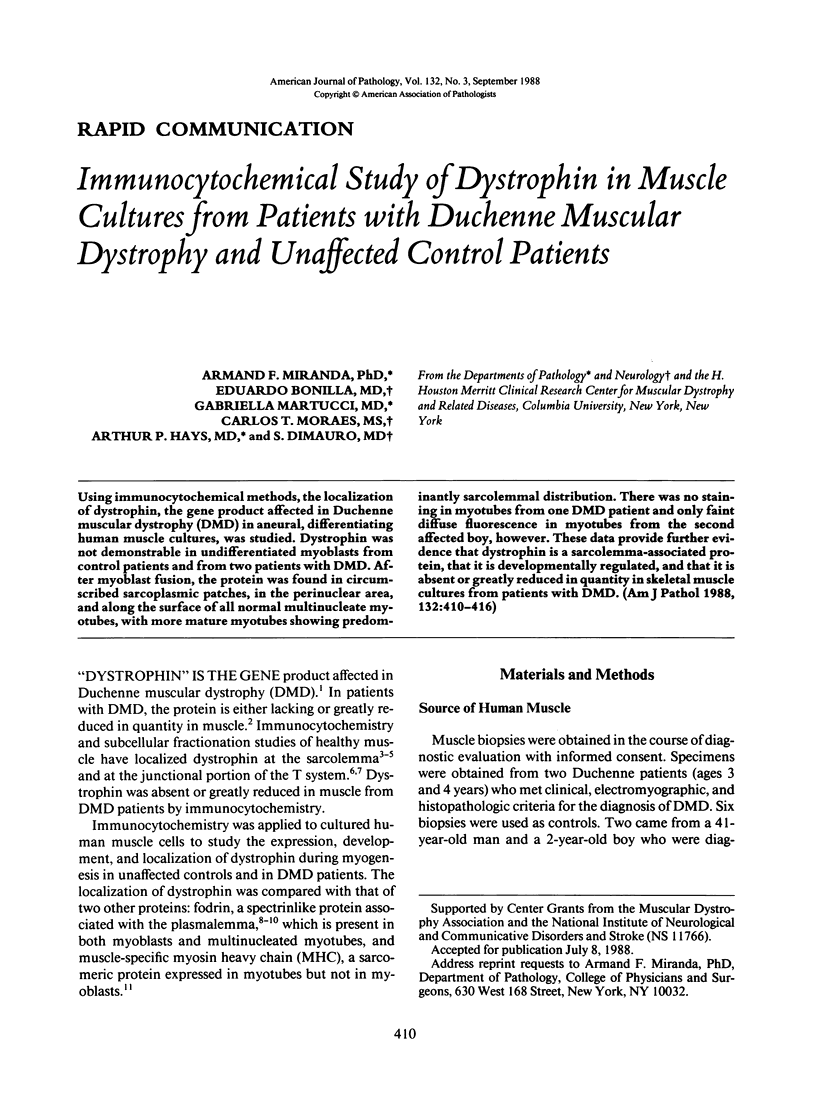


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard S. T., Dunn M. J., Dubowitz V., Scott M. L., Pittman S. J., Shotton D. M. Monoclonal antibodies detect a spectrin-like protein in normal and dystrophic human skeletal muscle. Proc Natl Acad Sci U S A. 1984 Feb;81(3):776–780. doi: 10.1073/pnas.81.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader D., Masaki T., Fischman D. A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982 Dec;95(3):763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M., Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Weber K. F-actin-binding and cross-linking properties of porcine brain fodrin, a spectrin-related molecule. J Biol Chem. 1982 Aug 25;257(16):9781–9787. [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Fischbeck K. H., Brown R. H., Johnson M., Medori R., Loike J. D., Harris J. B., Waterston R., Brooke M., Specht L. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne's or Becker's muscular dystrophy. N Engl J Med. 1988 May 26;318(21):1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Knudson C. M., Campbell K. P., Kunkel L. M. Subcellular fractionation of dystrophin to the triads of skeletal muscle. Nature. 1987 Dec 24;330(6150):754–758. doi: 10.1038/330754a0. [DOI] [PubMed] [Google Scholar]
- Lev A. A., Feener C. C., Kunkel L. M., Brown R. H., Jr Expression of the Duchenne's muscular dystrophy gene in cultured muscle cells. J Biol Chem. 1987 Nov 25;262(33):15817–15820. [PubMed] [Google Scholar]
- MAURO A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961 Feb;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawatari S., Miranda A., Rowland L. P. Adenyl cyclase abnormality in Duchenne muscular dystrophy: muscle cells in culture. Neurology. 1976 Nov;26(11):1021–1026. doi: 10.1212/wnl.26.11.1021. [DOI] [PubMed] [Google Scholar]
- Miranda A. F., Babiss L. E., Fisher P. B. Measurement of the effect of interferons on cellular differentiation of human skeletal muscle cells. Methods Enzymol. 1986;119:619–628. doi: 10.1016/0076-6879(86)19083-5. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Neve R. L., Colletti-Feener C., Bertelson C. J., Kurnit D. M., Kunkel L. M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986 Oct 16;323(6089):646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- Nudel U., Robzyk K., Yaffe D. Expression of the putative Duchenne muscular dystrophy gene in differentiated myogenic cell cultures and in the brain. Nature. 1988 Feb 18;331(6157):635–638. doi: 10.1038/331635a0. [DOI] [PubMed] [Google Scholar]
- Peterson E. R., Masurovsky E. B., Spiro A. J., Crain S. M. Duchenne dystrophic muscle develops lesions in long-term coculture with mouse spinal cord. Muscle Nerve. 1986 Nov-Dec;9(9):787–808. doi: 10.1002/mus.880090903. [DOI] [PubMed] [Google Scholar]
- Repasky E. A., Pollina C. M., Menold M. M., Hudecki M. S. Increased concentration of spectrin is observed in avian dystrophic muscle. Proc Natl Acad Sci U S A. 1986 Feb;83(3):802–806. doi: 10.1073/pnas.83.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. O., Sylvester J. E., Heiman-Patterson T., Shi Y. J., Fieles W., Stedman H., Burghes A., Ray P., Worton R., Fischbeck K. H. Duchenne muscular dystrophy gene expression in normal and diseased human muscle. Science. 1988 Mar 18;239(4846):1418–1420. doi: 10.1126/science.2450401. [DOI] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Bulman D. E., Karpati G., Burghes A. H., Belfall B., Klamut H. J., Talbot J., Hodges R. S., Ray P. N., Worton R. G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988 Jun 2;333(6172):466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]