Abstract
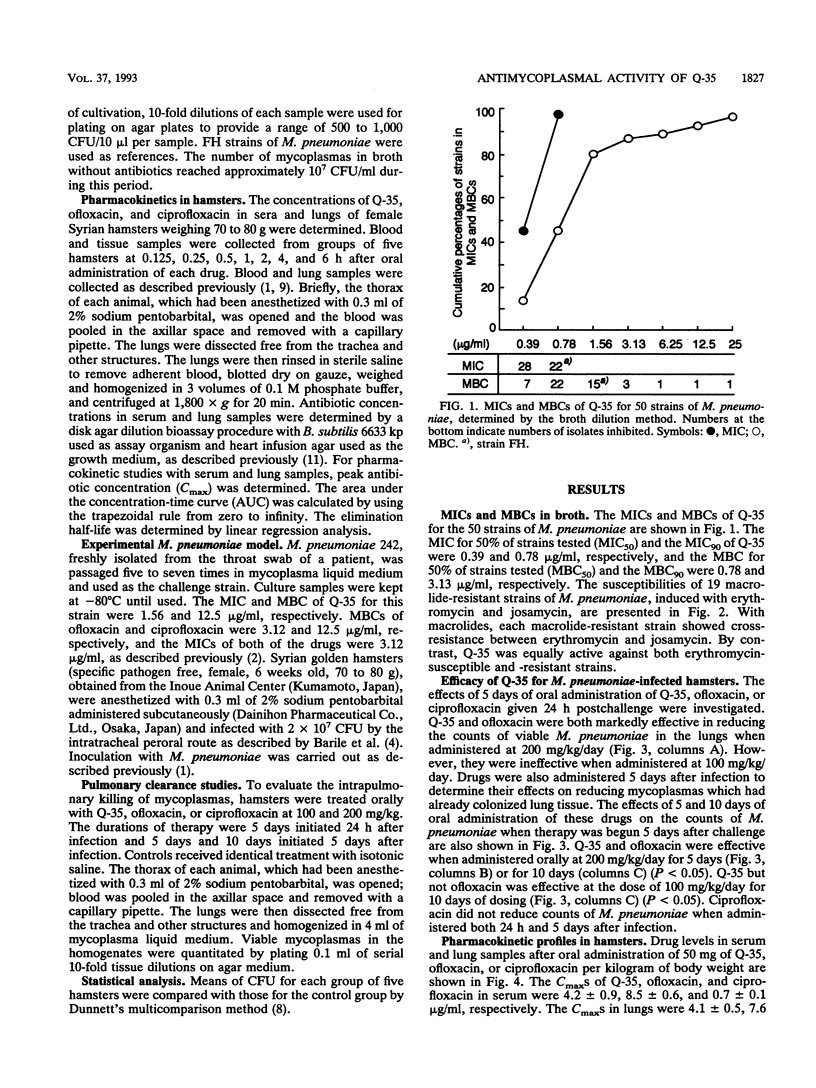
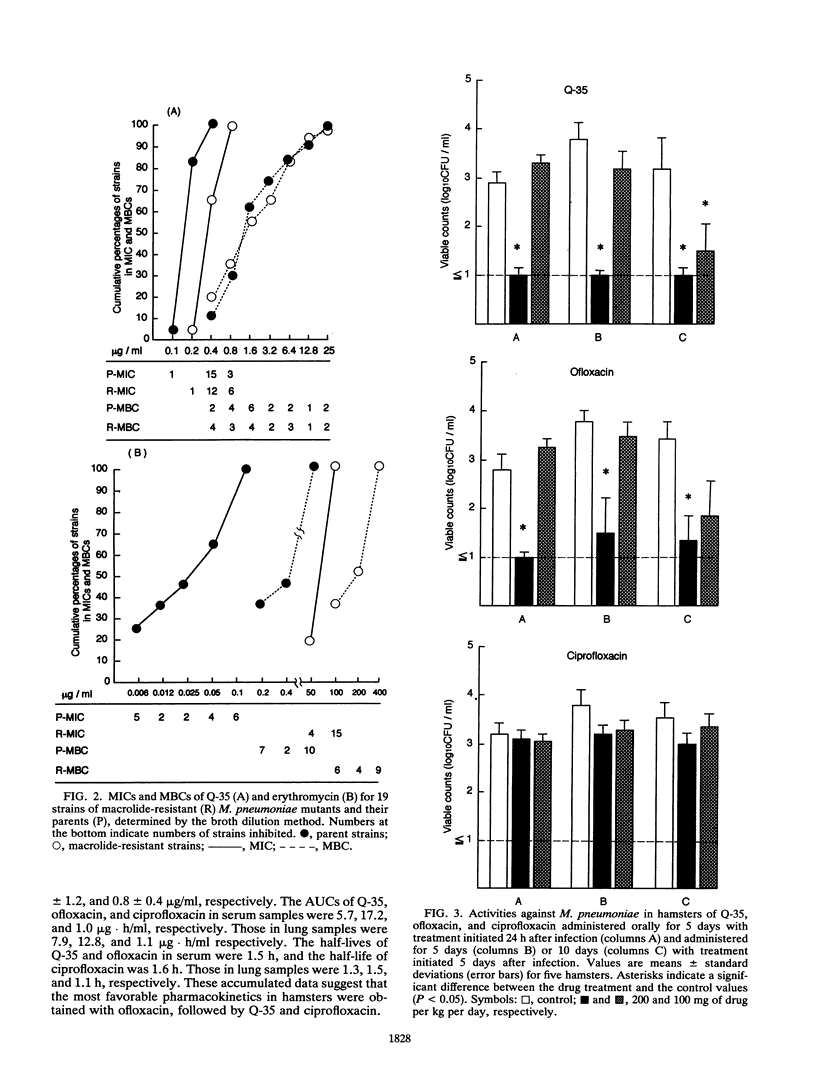
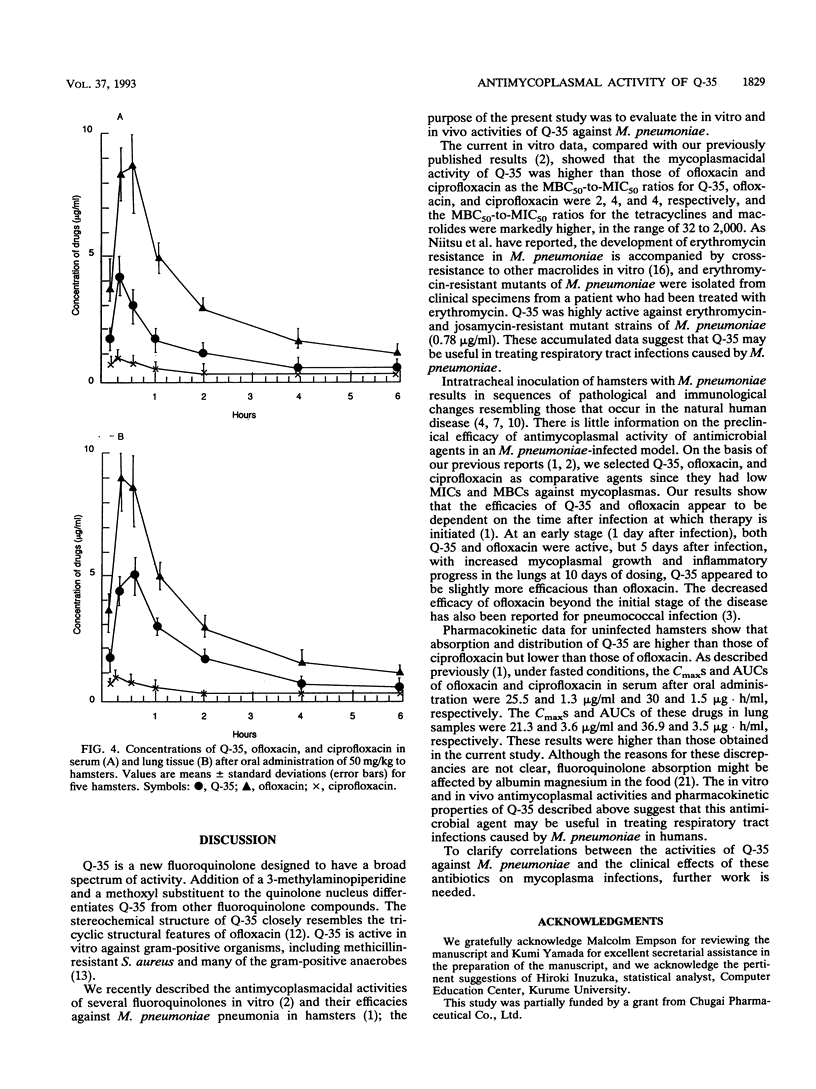
The in vitro potency and in vivo efficacy of Q-35, a new fluoroquinolone, against Mycoplasma pneumoniae were investigated by pharmacokinetic studies with M. pneumoniae-infected hamsters. By using fluoroquinolones, macrolides, and tetracyclines as references, Q-35 was found to possess the greatest mycoplasmacidal activity. The MIC for 90% of strains tested (MIC90) and the MIC50 were 0.78 and 0.39 microgram/ml, respectively, and the MBC for 90% of strains tested (MBC90) and the MBC50 were 3.13 and 0.78 microgram/ml, respectively. The MBC50-to-MIC50 ratio for Q-35 was 2. Furthermore, only Q-35 continued to be effective against 19 strains of erythromycin-resistant mutants of M. pneumoniae. The efficacies of fluoroquinolones against M. pneumoniae were also investigated by using an experimental hamster pneumonia model to measure the CFU of M. pneumoniae in the lungs. Q-35 and ofloxacin were efficacious following oral administration of 200 mg/kg/day for 5 days, initiated 24 h after infection, while ciprofloxacin was not active. Continuous administration of Q-35 for 10 days significantly reduced numbers of viable M. pneumoniae in the lungs. These results suggest that both Q-35 and ofloxacin are effective in the early phase of infection and, moreover, that Q-35 is also effective in the middle stage of infection, when progressive lung alterations and continuous increases in mycoplasmal growth occur. Peak levels of Q-35 in sera and lungs after oral administration were higher than those of ciprofloxacin but lower than those of ofloxacin. On the basis of these results, Q-35 appears to be a promising antimicrobial agent in chemotherapy of mycoplasmal infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai S., Gohara Y., Akashi A., Kuwano K., Nishimoto M., Yano T., Oizumi K., Takeda K., Yamaguchi T. Effects of new quinolones on Mycoplasma pneumoniae-infected hamsters. Antimicrob Agents Chemother. 1993 Feb;37(2):287–292. doi: 10.1128/aac.37.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S., Gohara Y., Kuwano K., Kawashima T. Antimycoplasmal activities of new quinolones, tetracyclines, and macrolides against Mycoplasma pneumoniae. Antimicrob Agents Chemother. 1992 Jun;36(6):1322–1324. doi: 10.1128/aac.36.6.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay-Dupuis E., Bedos J. P., Vallée E., Hardy D. J., Swanson R. N., Pocidalo J. J. Antipneumococcal activity of ciprofloxacin, ofloxacin, and temafloxacin in an experimental mouse pneumonia model at various stages of the disease. J Infect Dis. 1991 Feb;163(2):319–324. doi: 10.1093/infdis/163.2.319. [DOI] [PubMed] [Google Scholar]
- Barile M. F., Chandler D. K., Yoshida H., Grabowski M. W., Harasawa R., Razin S. Parameters of Mycoplasma pneumoniae infection in Syrian hamsters. Infect Immun. 1988 Sep;56(9):2443–2449. doi: 10.1128/iai.56.9.2443-2449.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H., Waites K. B., Pate M. S., Canupp K. C., Duffy L. B. Comparative susceptibility of Mycoplasma pneumoniae to erythromycin, ciprofloxacin, and lomefloxacin. Diagn Microbiol Infect Dis. 1989 Sep-Oct;12(5):433–435. doi: 10.1016/0732-8893(89)90115-6. [DOI] [PubMed] [Google Scholar]
- Clyde W. A., Jr Immunopathology of experimental Mycoplasma pneumoniae disease. Infect Immun. 1971 Dec;4(6):757–763. doi: 10.1128/iai.4.6.757-763.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein P. H., Edelstein M. A., Weidenfeld J., Dorr M. B. In vitro activity of sparfloxacin (CI-978; AT-4140) for clinical Legionella isolates, pharmacokinetics in guinea pigs, and use to treat guinea pigs with L. pneumophila pneumonia. Antimicrob Agents Chemother. 1990 Nov;34(11):2122–2127. doi: 10.1128/aac.34.11.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald G. W., Clyde W. A., Jr, Bienenstock J. Immunoglobulin-containing cells in lungs of hamsters infected with Mycoplasma pneumoniae. J Immunol. 1972 May;108(5):1400–1408. [PubMed] [Google Scholar]
- Fernandes P. B., Chu D. T., Bower R. R., Jarvis K. P., Ramer N. R., Shipkowitz N. In vivo evaluation of A-56619 (difloxacin) and A-56620: new aryl-fluoroquinolones. Antimicrob Agents Chemother. 1986 Feb;29(2):201–208. doi: 10.1128/aac.29.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets L., Lindholm-Levy P. Comparison of bactericidal activities of streptomycin, amikacin, kanamycin, and capreomycin against Mycobacterium avium and M. tuberculosis. Antimicrob Agents Chemother. 1989 Aug;33(8):1298–1301. doi: 10.1128/aac.33.8.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Otsuki M., Nishino T. In vitro antibacterial activity of Q-35, a new fluoroquinolone. Antimicrob Agents Chemother. 1992 Aug;36(8):1708–1714. doi: 10.1128/aac.36.8.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Kojima K., Nagano H., Matsubara S., Yokota T. Photostability and biological activity of fluoroquinolones substituted at the 8 position after UV irradiation. Antimicrob Agents Chemother. 1992 Aug;36(8):1715–1719. doi: 10.1128/aac.36.8.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misu T., Arai S., Furukawa M., Yamamoto Y., Miyazaki T. Effects of rokitamycin and other macrolide antibiotics on Mycoplasma pneumoniae in L cells. Antimicrob Agents Chemother. 1987 Nov;31(11):1843–1845. doi: 10.1128/aac.31.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niitu Y., Hasegawa S., Suetake T., Kubota H., Komatsu S., Horikawa M. Resistance of Mycoplasma pneumoniae to erythromycin and other antibiotics. J Pediatr. 1970 Mar;76(3):438–443. doi: 10.1016/s0022-3476(70)80485-1. [DOI] [PubMed] [Google Scholar]
- Nitu Y., Hasegawa S., Kubota H. In vitro development of resistance to erythromycin, other macrolide antibiotics, and lincomycin in Mycoplasma pneumoniae. Antimicrob Agents Chemother. 1974 May;5(5):513–519. doi: 10.1128/aac.5.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada Y., Ogawa H. Antimycoplasmal activity of ofloxacin (DL-8280). Antimicrob Agents Chemother. 1983 Mar;23(3):509–511. doi: 10.1128/aac.23.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgel F., Naber K. G., Kinzig M., Mahr G., Muth P. Comparative pharmacokinetics of ciprofloxacin and temafloxacin in humans: a review. Am J Med. 1991 Dec 30;91(6A):51S–66S. doi: 10.1016/0002-9343(91)90312-l. [DOI] [PubMed] [Google Scholar]
- Stanbridge E., Hayflick L. Growth inhibition test for identification of Mycoplasma species utilizing dried antiserum-impregnated paper discs. J Bacteriol. 1967 Apr;93(4):1392–1396. doi: 10.1128/jb.93.4.1392-1396.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. C., Schoenknecht F. D., Sherris J. C., Linner E. C. Determination of minimum bactericidal concentrations of oxacillin for Staphylococcus aureus: influence and significance of technical factors. Antimicrob Agents Chemother. 1983 Jan;23(1):142–150. doi: 10.1128/aac.23.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]



