Abstract
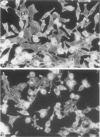
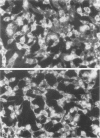
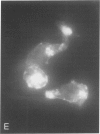
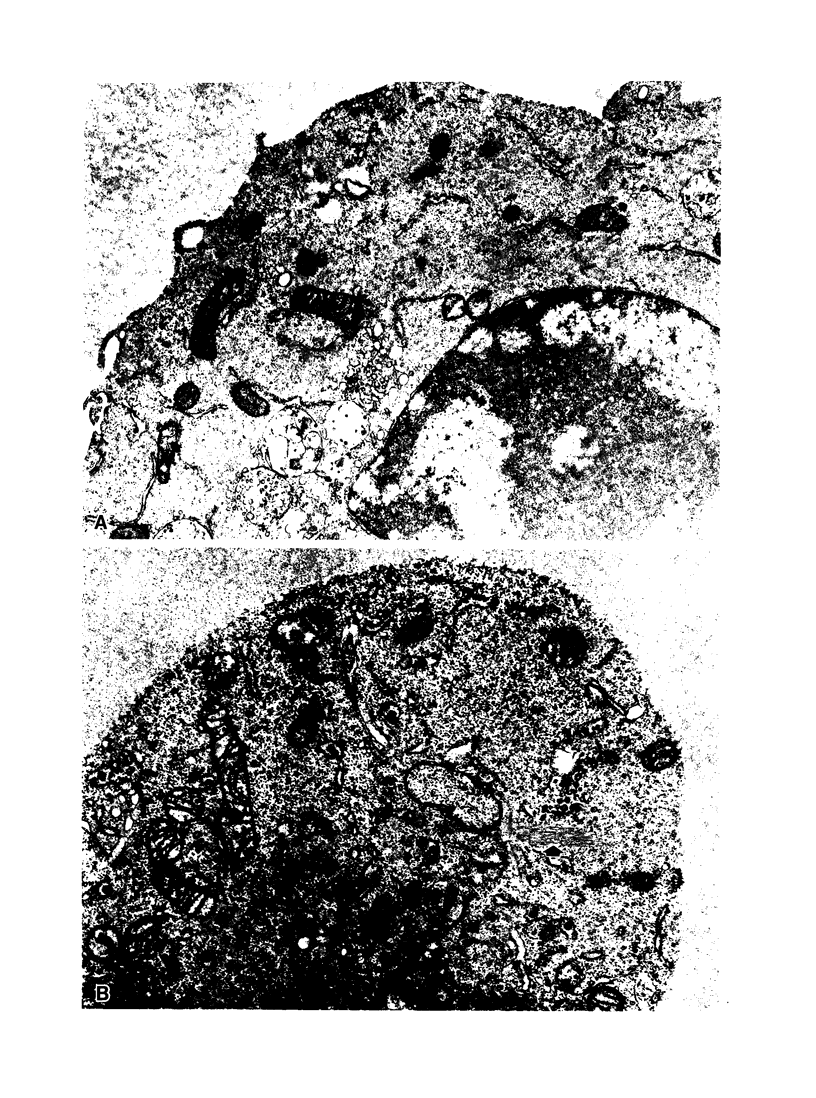
Oxidant injury produces dramatic changes in cytoskeletal organization and cell shape. ATP synthetic pathways are major targets of oxidant injury resulting in rapid depletion of cellular ATP following oxidant exposure. The relation of ATP depletion to the changes in microfilament organization seen following H2O2 exposure were examined in the P388D1 cell line. Three hours of glucose depletion alone resulted in a decline in cellular ATP levels to less than 10% of controls, which was comparable to ATP levels in cells 30 to 60 minutes after exposure to 5 mM H2O2 in the presence of glucose. Adherent cells stained with rhodamine phalloidin, a probe specific for polymerized (F) actin, revealed a progressive shortening of microfilaments into globular aggregates within cells depleted of glucose over 3 hours, a pattern similar to earlier observations of H2O2-injured cells after 1 hour. The changes in cellular ATP associated with glucose depletion or H2O2 exposure were then correlated with G actin content measured by the DNAse 1 inhibition assay. No real differences in G actin content as a percentage of total actin were seen in P388D1 cells following 3 hours of glucose depletion or 30 to 60 minutes after exposure to 5 mM H2O2. But 2 to 3 hours after exposure to H2O2 there was a progressive decrease in G actin as a percentage of total actin within the cells. Transmission electron microscopy of cells depleted of glucose for 3 h or 1 hour after exposure to H2O2 revealed the presence of side-to-side aggregates or bundles of microfilaments within the cells. These observations suggest that declining levels of ATP either from metabolic inhibition or H2O2 injury are correlated with the fragmentation and shortening of microfilaments into aggregates. No net change in monomeric or polymeric actin was necessary for this to occur. However, at later time points after H2O2 exposure some actin assembly did occur.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bereiter-Hahn J., Tillmann U., Vöth M. Interaction of metabolic inhibitors with actin fibrils. Cell Tissue Res. 1984;238(1):129–134. doi: 10.1007/BF00215153. [DOI] [PubMed] [Google Scholar]
- Bershadsky A. D., Gelfand V. I. Role of ATP in the regulation of stability of cytoskeletal structures. Cell Biol Int Rep. 1983 Mar;7(3):173–187. doi: 10.1016/0309-1651(83)90218-7. [DOI] [PubMed] [Google Scholar]
- Bershadsky A. D., Gelfand V. I., Svitkina T. M., Tint I. S. Destruction of microfilament bundles in mouse embryo fibroblasts treated with inhibitors of energy metabolism. Exp Cell Res. 1980 Jun;127(2):421–429. doi: 10.1016/0014-4827(80)90446-2. [DOI] [PubMed] [Google Scholar]
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Hinshaw D. B., Armstrong B. C., Beals T. F., Hyslop P. A. A cellular model of endothelial cell ischemia. J Surg Res. 1988 May;44(5):527–537. doi: 10.1016/0022-4804(88)90158-8. [DOI] [PubMed] [Google Scholar]
- Hinshaw D. B., Sklar L. A., Bohl B., Schraufstatter I. U., Hyslop P. A., Rossi M. W., Spragg R. G., Cochrane C. G. Cytoskeletal and morphologic impact of cellular oxidant injury. Am J Pathol. 1986 Jun;123(3):454–464. [PMC free article] [PubMed] [Google Scholar]
- Howard T. H., Meyer W. H. Chemotactic peptide modulation of actin assembly and locomotion in neutrophils. J Cell Biol. 1984 Apr;98(4):1265–1271. doi: 10.1083/jcb.98.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop P. A., Hinshaw D. B., Halsey W. A., Jr, Schraufstätter I. U., Sauerheber R. D., Spragg R. G., Jackson J. H., Cochrane C. G. Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J Biol Chem. 1988 Feb 5;263(4):1665–1675. [PubMed] [Google Scholar]
- Hyslop P. A., Hinshaw D. B., Schraufstätter I. U., Sklar L. A., Spragg R. G., Cochrane C. G. Intracellular calcium homeostasis during hydrogen peroxide injury to cultured P388D1 cells. J Cell Physiol. 1986 Dec;129(3):356–366. doi: 10.1002/jcp.1041290314. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Martin W. J., 2nd Neutrophils kill pulmonary endothelial cells by a hydrogen-peroxide-dependent pathway. An in vitro model of neutrophil-mediated lung injury. Am Rev Respir Dis. 1984 Aug;130(2):209–213. doi: 10.1164/arrd.1984.130.2.209. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Sanger J. M., Jockusch B. M. Differential response of three types of actin filament bundles to depletion of cellular ATP levels. Eur J Cell Biol. 1983 Sep;31(2):197–204. [PubMed] [Google Scholar]
- Schraufstatter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986 Apr;77(4):1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hyslop P. A., Hinshaw D. B., Spragg R. G., Sklar L. A., Cochrane C. G. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4908–4912. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstätter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Glutathione cycle activity and pyridine nucleotide levels in oxidant-induced injury of cells. J Clin Invest. 1985 Sep;76(3):1131–1139. doi: 10.1172/JCI112068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Spragg R. G., Hinshaw D. B., Hyslop P. A., Schraufstätter I. U., Cochrane C. G. Alterations in adenosine triphosphate and energy charge in cultured endothelial and P388D1 cells after oxidant injury. J Clin Invest. 1985 Oct;76(4):1471–1476. doi: 10.1172/JCI112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland T., Faulstich H. Amatoxins, phallotoxins, phallolysin, and antamanide: the biologically active components of poisonous Amanita mushrooms. CRC Crit Rev Biochem. 1978 Dec;5(3):185–260. doi: 10.3109/10409237809149870. [DOI] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]