Abstract
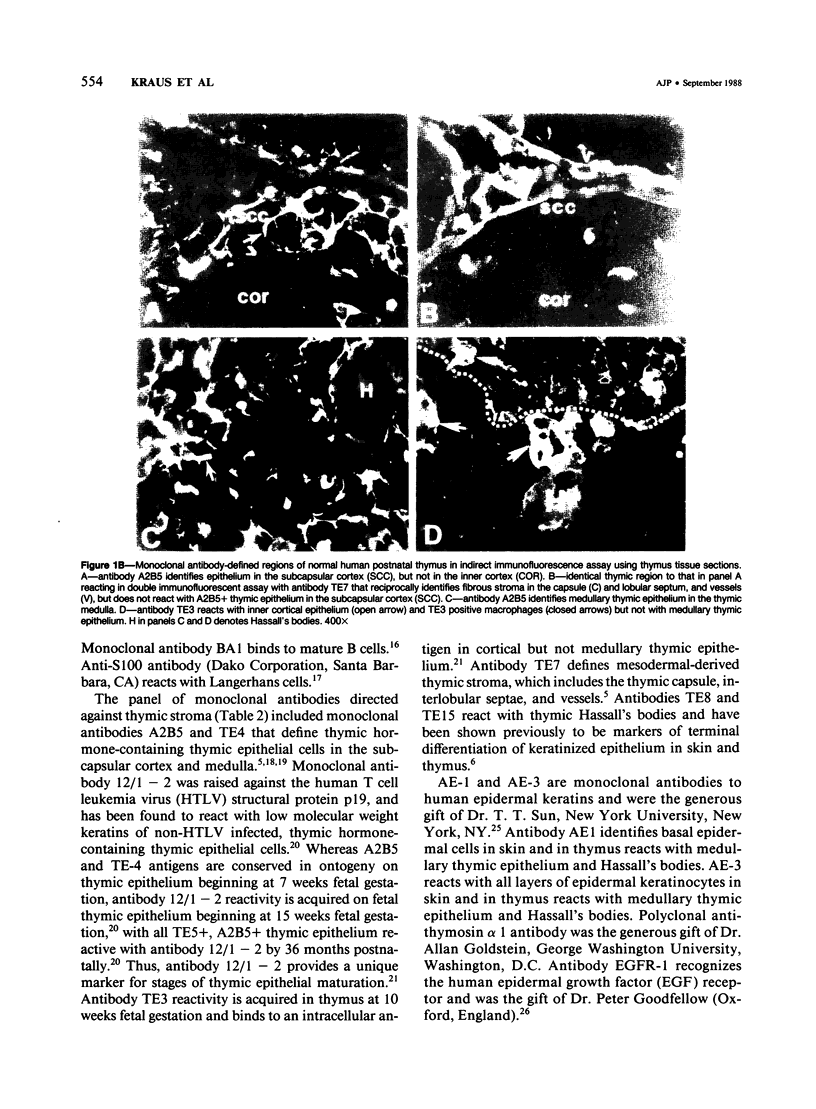
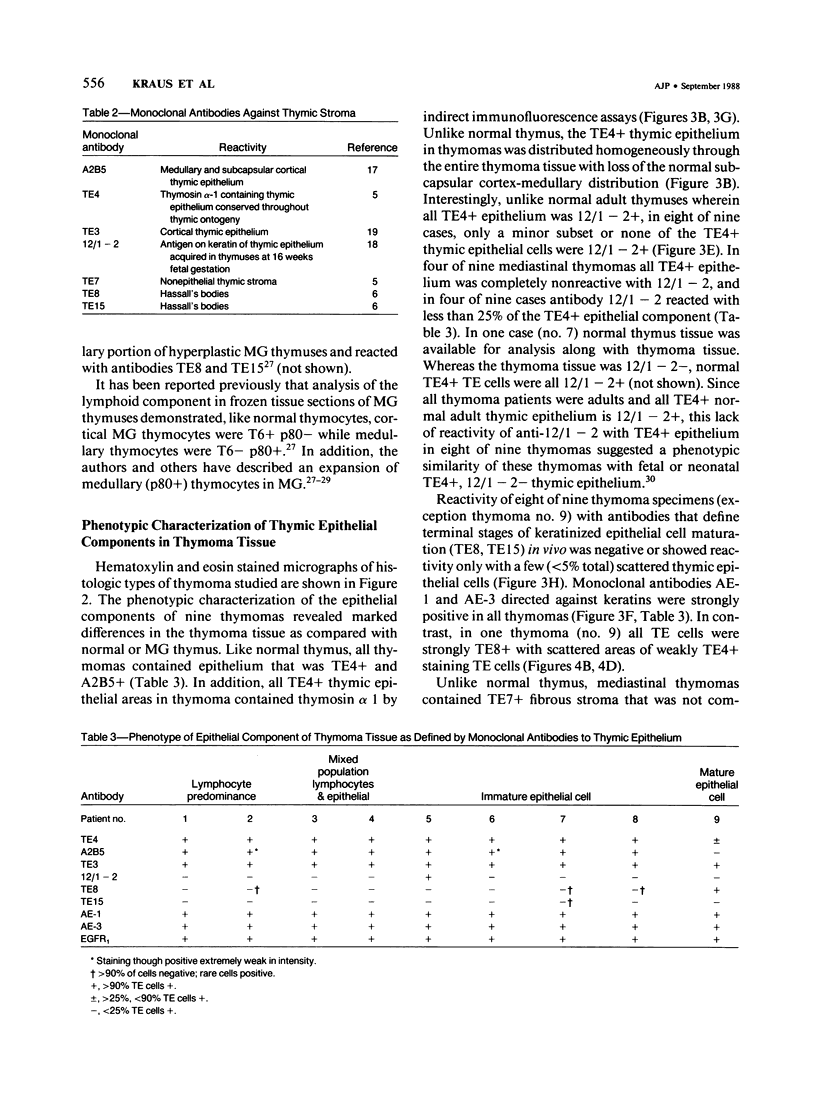
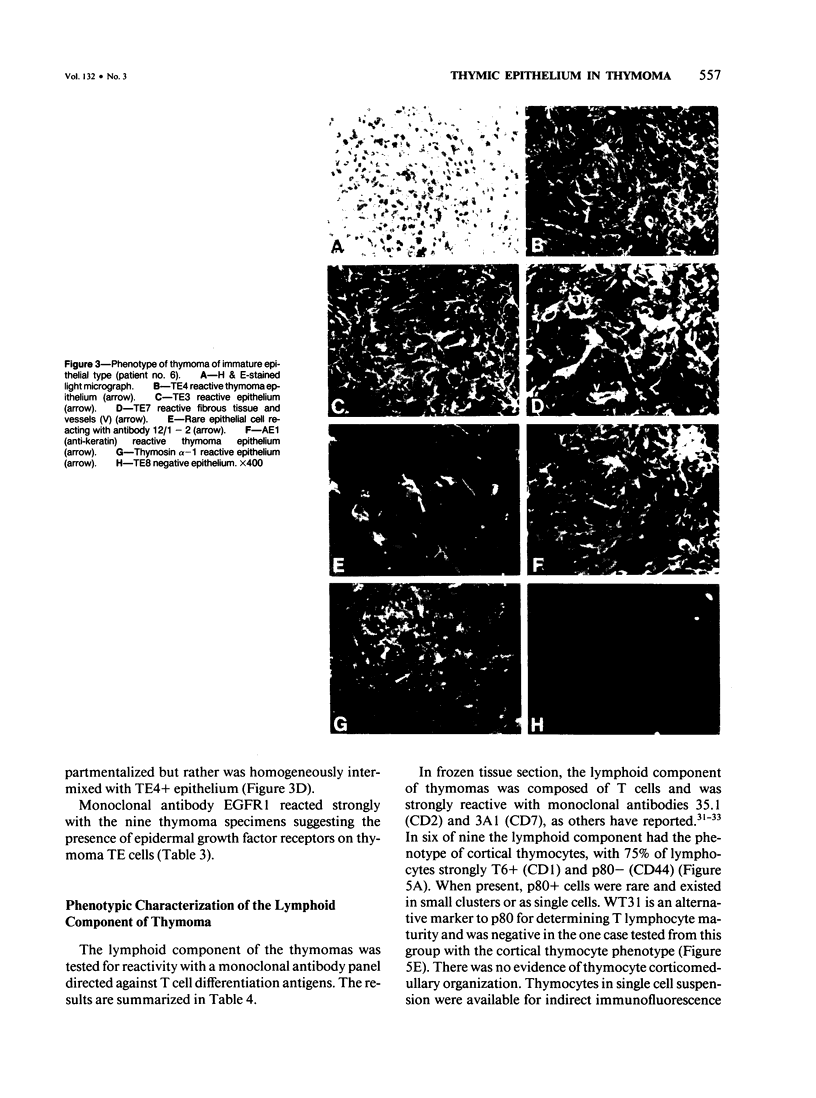
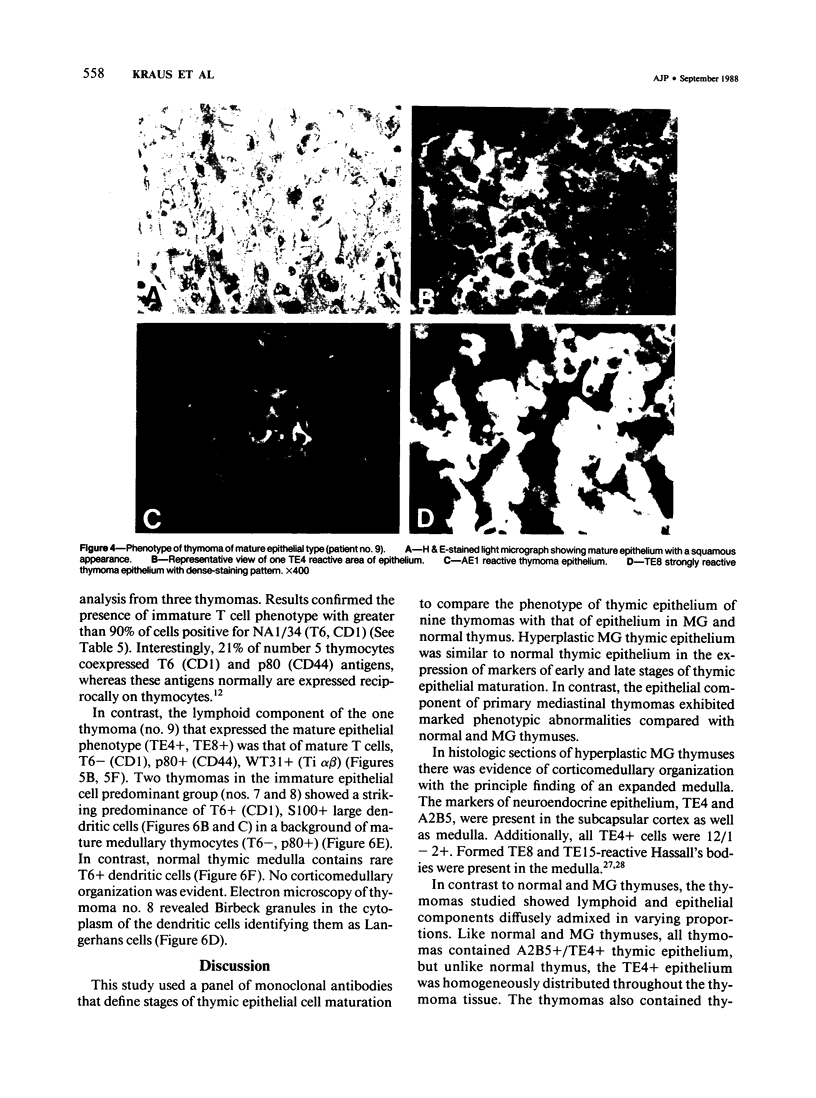
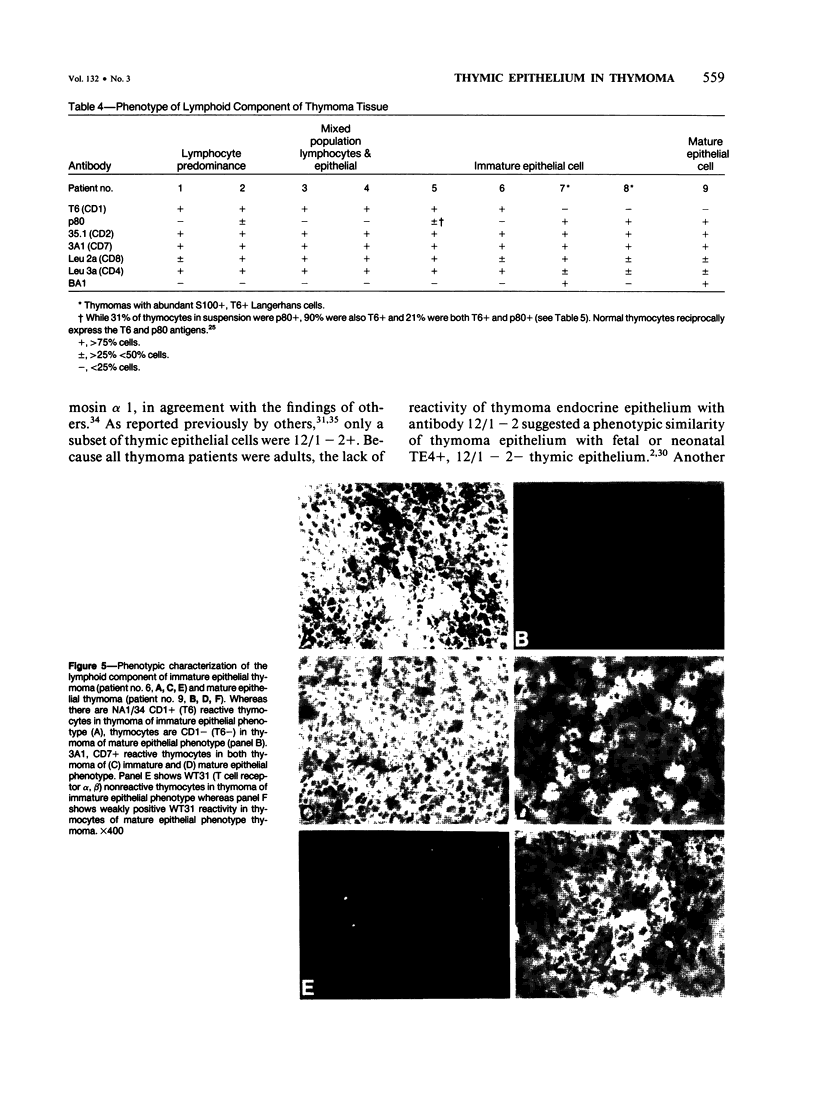
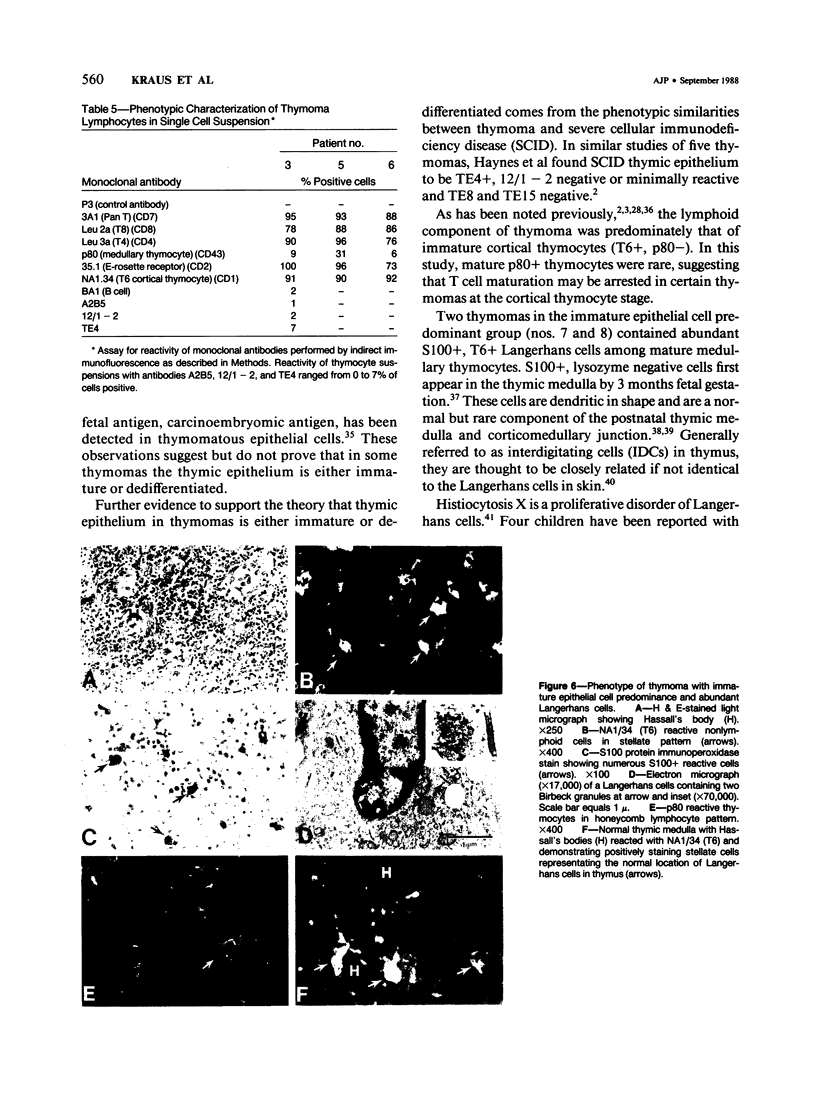
A panel of monoclonal antibodies that phenotypically define stages of normal human thymic epithelial (TE) cell maturation was used to compare thymic epithelium of nine thymomas with hyperplastic thymic epithelium in myasthenia gravis (MG) and thymic epithelium of normal thymuses. It has been shown previously that normal thymic epithelial cells express antigens of early TE cell maturation (A2B5, TE-4) throughout thymic ontogeny and acquire antigens 12/1-2, TE8, and TE-15 at 14 to 16 weeks of fetal gestation. Hyperplastic MG thymic epithelial cells expressed TE antigens in phenotypic patterns similar to that seen in normal postnatal thymus, ie, TE in subcapsular cortex and medulla was TE4+, A2B5+, and 12/1 - 2+ and Hassall's bodies were reactive with antibodies TE8 and TE15. In contrast, thymic epithelium in primary mediastinal thymomas was TE4+, A2B5+, TE8-, and greater than 75% of thymoma epithelium was 12/1 - 2-, a thymic epithelial phenotype similar to that seen on normal fetal thymic epithelium at 14 to 16 weeks fetal gestation. In one subject with a mature epithelial histologic pattern, thymoma epithelium was found to be strongly TE8+, a phenotype suggestive of a later stage of TE maturation. Lymphocytes in five of seven thymomas with immature thymic epithelial cells predominantly expressed immature thymocyte phenotype while two thymomas with immature epithelial phenotype showed a predominance of Langerhans cells and surrounding lymphocytes expressing a mature phenotype. Lymphocytes in the thymoma with differentiated epithelial cells expressed a mature thymocyte phenotype. Thus, in thymomas of varying histologic types, phenotypic abnormalities of thymic epithelium are present; these phenotypic abnormalities may reflect abnormal thymic epithelial maturation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson C. S., Kersey J. H., LeBien T. W. A monoclonal antibody (BA-1) reactive with cells of human B lymphocyte lineage. J Immunol. 1981 Jan;126(1):83–88. [PubMed] [Google Scholar]
- Beckstead J. H., Wood G. S., Turner R. R. Histiocytosis X cells and Langerhans cells: enzyme histochemical and immunologic similarities. Hum Pathol. 1984 Sep;15(9):826–833. doi: 10.1016/s0046-8177(84)80143-4. [DOI] [PubMed] [Google Scholar]
- Bramwell N. H., Burns B. F. Histiocytosis X of the thymus in association with myasthenia gravis. Am J Clin Pathol. 1986 Aug;86(2):224–227. doi: 10.1093/ajcp/86.2.224. [DOI] [PubMed] [Google Scholar]
- Chilosi M., Iannucci A. M., Pizzolo G., Menestrina F., Fiore-Donati L., Janossy G. Immunohistochemical analysis of thymoma. Evidence for medullary origin of epithelial cells. Am J Surg Pathol. 1984 Apr;8(4):309–318. doi: 10.1097/00000478-198404000-00009. [DOI] [PubMed] [Google Scholar]
- Chilosi M., Iannucci A., Fiore-Donati L., Tridente G., Pampanin M., Pizzolo G., Ritter M., Bofill M., Janossy G. Myasthenia gravis: immunohistological heterogeneity in microenvironmental organization of hyperplastic and neoplastic thymuses suggesting different mechanisms of tolerance breakdown. J Neuroimmunol. 1986 May;11(3):191–204. doi: 10.1016/0165-5728(86)90003-2. [DOI] [PubMed] [Google Scholar]
- Eimoto T., Teshima K., Shirakusa T., Takeshita M., Okamura H., Naito H., Mitsui T., Kikuchi M. Heterogeneity of epithelial cells and reactive components in thymomas: an ultrastructural and immunohistochemical study. Ultrastruct Pathol. 1986;10(2):157–173. doi: 10.3109/01913128609014592. [DOI] [PubMed] [Google Scholar]
- Favara B. E., McCarthy R. C., Mierau G. W. Histiocytosis X. Hum Pathol. 1983 Aug;14(8):663–676. doi: 10.1016/s0046-8177(83)80138-5. [DOI] [PubMed] [Google Scholar]
- Hamoudi A. B., Newton W. A., Jr, Mancer K., Penn G. M. Thymic changes in histiocytosis. Am J Clin Pathol. 1982 Feb;77(2):169–173. doi: 10.1093/ajcp/77.2.169. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Eisenbarth G. S., Fauci A. S. Human lymphocyte antigens: production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5829–5833. doi: 10.1073/pnas.76.11.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Harden E. A., Telen M. J., Hemler M. E., Strominger J. L., Palker T. J., Scearce R. M., Eisenbarth G. S. Differentiation of human T lymphocytes. I. Acquisition of a novel human cell surface protein (p80) during normal intrathymic T cell maturation. J Immunol. 1983 Sep;131(3):1195–1200. [PubMed] [Google Scholar]
- Haynes B. F., Hensley L. L., Jegasothy B. V. Differentiation of human T lymphocytes: II. Phenotypic difference in skin and blood malignant T-cells in cutaneous T-cell lymphoma. J Invest Dermatol. 1982 Apr;78(4):323–326. doi: 10.1111/1523-1747.ep12507406. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Metzgar R. S., Minna J. D., Bunn P. A. Phenotypic characterization of cutaneous T-cell lymphoma. Use of monoclonal antibodies to compare with other malignant T cells. N Engl J Med. 1981 May 28;304(22):1319–1323. doi: 10.1056/NEJM198105283042202. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Robert-Guroff M., Metzgar R. S., Franchini G., Kalyanaraman V. S., Palker T. J., Gallo R. C. Monoclonal antibody against human T cell leukemia virus p19 defines a human thymic epithelial antigen acquired during ontogeny. J Exp Med. 1983 Mar 1;157(3):907–920. doi: 10.1084/jem.157.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Scearce R. M., Lobach D. F., Hensley L. L. Phenotypic characterization and ontogeny of mesodermal-derived and endocrine epithelial components of the human thymic microenvironment. J Exp Med. 1984 Apr 1;159(4):1149–1168. doi: 10.1084/jem.159.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F. The human thymic microenvironment. Adv Immunol. 1984;36:87–142. doi: 10.1016/s0065-2776(08)60900-1. [DOI] [PubMed] [Google Scholar]
- Haynes B. F. The role of the thymic microenvironment in promotion of early stages of human T cell maturation. Clin Res. 1986 Sep;34(3):422–431. [PubMed] [Google Scholar]
- Kornstein M. J., Hoxie J. A., Levinson A. I., Brooks J. J. Immunohistology of human thymomas. Arch Pathol Lab Med. 1985 May;109(5):460–463. [PubMed] [Google Scholar]
- Laster A. J., Itoh T., Palker T. J., Haynes B. F. The human thymic microenvironment: thymic epithelium contains specific keratins associated with early and late stages of epidermal keratinocyte maturation. Differentiation. 1986;31(1):67–77. doi: 10.1111/j.1432-0436.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Lauriola L., Michetti F., Stolfi V. M., Tallini G., Cocchia D. Detection by S-100 immunolabelling of interdigitating reticulum cells in human thymomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(2):187–195. doi: 10.1007/BF02889864. [DOI] [PubMed] [Google Scholar]
- Levine G. D., Bensch K. G. Epithelial nature of spindle-cell thymoma. An ultrastructural study. Cancer. 1972 Aug;30(2):500–511. doi: 10.1002/1097-0142(197208)30:2<500::aid-cncr2820300230>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Levine G. D., Rosai J. Thymic hyperplasia and neoplasia: a review of current concepts. Hum Pathol. 1978 Sep;9(5):495–515. doi: 10.1016/s0046-8177(78)80131-2. [DOI] [PubMed] [Google Scholar]
- Lobach D. F., Haynes B. F. Ontogeny of the human thymus during fetal development. J Clin Immunol. 1987 Mar;7(2):81–97. doi: 10.1007/BF00916002. [DOI] [PubMed] [Google Scholar]
- Lobach D. F., Itoh T., Singer K. H., Haynes B. F. The thymic microenvironment. Characterization of in vitro differentiation of the IT26R21 rat thymic epithelial cell line. Differentiation. 1987;34(1):50–59. doi: 10.1111/j.1432-0436.1987.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Lobach D. F., Scearce R. M., Haynes B. F. The human thymic microenvironment. Phenotypic characterization of Hassall's bodies with the use of monoclonal antibodies. J Immunol. 1985 Jan;134(1):250–257. [PubMed] [Google Scholar]
- Martin P. J., Longton G., Ledbetter J. A., Newman W., Braun M. P., Beatty P. G., Hansen J. A. Identification and functional characterization of two distinct epitopes on the human T cell surface protein Tp50. J Immunol. 1983 Jul;131(1):180–185. [PubMed] [Google Scholar]
- McFarland E. J., Scearce R. M., Haynes B. F. The human thymic microenvironment: cortical thymic epithelium is an antigenically distinct region of the thymic microenvironment. J Immunol. 1984 Sep;133(3):1241–1249. [PubMed] [Google Scholar]
- McMichael A. J., Pilch J. R., Galfré G., Mason D. Y., Fabre J. W., Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979 Mar;9(3):205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- Mokhtar N., Hsu S. M., Lad R. P., Haynes B. F., Jaffe E. S. Thymoma: lymphoid and epithelial components mirror the phenotype of normal thymus. Hum Pathol. 1984 Apr;15(4):378–384. doi: 10.1016/s0046-8177(84)80037-4. [DOI] [PubMed] [Google Scholar]
- Müller-Hermelink H. K., Marino M., Palestro G. Pathology of thymic epithelial tumors. Curr Top Pathol. 1986;75:207–268. doi: 10.1007/978-3-642-82480-7_7. [DOI] [PubMed] [Google Scholar]
- Pelletier M., Tautu C., Landry D., Montplaisir S., Chartrand C., Perreault C. Characterization of human thymic dendritic cells in culture. Immunology. 1986 Jun;58(2):263–270. [PMC free article] [PubMed] [Google Scholar]
- Savino W., Dardenne M. Thymic hormone-containing cells VI. Immunohistologic evidence for the simultaneous presence of thymulin, thymopoietin and thymosin alpha 1 in normal and pathological human thymuses. Eur J Immunol. 1984 Nov;14(11):987–991. doi: 10.1002/eji.1830141105. [DOI] [PubMed] [Google Scholar]
- Savino W., Durand D., Dardenne M. Immunohistochemical evidence for the expression of the carcinoembryonic antigen by human thymic epithelial cells in vitro and in neoplastic conditions. Am J Pathol. 1985 Dec;121(3):418–425. [PMC free article] [PubMed] [Google Scholar]
- Siegal G. P., Dehner L. P., Rosai J. Histiocytosis X (Langerhans' cell granulomatosis) of the thymus. A clinicopathologic study of four childhood cases. Am J Surg Pathol. 1985 Feb;9(2):117–124. doi: 10.1097/00000478-198502000-00006. [DOI] [PubMed] [Google Scholar]
- Spits H., Borst J., Tax W., Capel P. J., Terhorst C., de Vries J. E. Characteristics of a monoclonal antibody (WT-31) that recognizes a common epitope on the human T cell receptor for antigen. J Immunol. 1985 Sep;135(3):1922–1928. [PubMed] [Google Scholar]
- Stutman O. Intrathymic and extrathymic T cell maturation. Immunol Rev. 1978;42:138–184. doi: 10.1111/j.1600-065x.1978.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Takacs L., Savino W., Monostori E., Ando I., Bach J. F., Dardenne M. Cortical thymocyte differentiation in thymomas: an immunohistologic analysis of the pathologic microenvironment. J Immunol. 1987 Feb 1;138(3):687–698. [PubMed] [Google Scholar]
- Takahashi K., Isobe T., Ohtsuki Y., Sonobe H., Takeda I., Akagi T. Immunohistochemical localization and distribution of S-100 proteins in the human lymphoreticular system. Am J Pathol. 1984 Sep;116(3):497–503. [PMC free article] [PubMed] [Google Scholar]
- Telen M. J., Shehata H., Haynes B. F. Human medullary thymocyte p80 antigen and In(Lu)-related p80 antigen reside on the same protein. Hum Immunol. 1986 Nov;17(3):311–324. doi: 10.1016/0198-8859(86)90283-1. [DOI] [PubMed] [Google Scholar]
- Thorbecke G. J., Silberberg-Sinakin I., Flotte T. J. Langerhans cells as macrophages in skin and lymphoid organs. J Invest Dermatol. 1980 Jul;75(1):32–43. doi: 10.1111/1523-1747.ep12521083. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Nakajima T., Shimosato Y., Shimamura K., Sakuma H. T-zone histiocytes with S100 protein. Development and distribution in human fetuses. Acta Pathol Jpn. 1983 Jan;33(1):15–22. [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Eichner R., Nelson W. G., Sun T. T. Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):580–588. doi: 10.1083/jcb.95.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]