Abstract
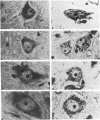
To evaluate the use of an anti-organelle antibody in a pathologic reaction the chromatolytic reaction was chosen for study. Qualitative analysis was made of rat hypoglossal nuclei stained with a cresyl violet method for Nissl substance and a monoclonal antibody against rat Golgi apparatus (10A8) 0 and 3 days, and 1, 2, 3, 4, 5, and 6 weeks after section of the right hypoglossal nerve. Marked dispersion of Nissl substance was noted at 2 weeks and of Golgi apparatus at 4 weeks. Reaggregation of staining had occurred for both organelles by 6 weeks. A quantitative light microscopic analysis was carried out for both stains on randomly selected hypoglossal sections from 0, 2, 4, and 6 weeks. The analysis confirmed the qualitative observations and showed them to be highly significant. In addition, it revealed an increase in nuclear area from 0 to 2 weeks, an increase in cytoplasmic area at 4 weeks, decrease in the total area of Nissl substance from 0 to 2 and 4 to 6 weeks, decrease in the percent cytoplasmic areas occupied by Nissl from 0 to 2 weeks and decreases in both the total and percent cytoplasmic area occupied by Golgi apparatus maximal at 4 weeks. Both qualitative and quantitative analyses confirmed the use of this monoclonal antibody to study morphologic changes of the Golgi apparatus secondary to an experimental pathologic lesion. In addition, a previously unrecognized temporal dissociation between the changes of Nissl substance and Golgi apparatus was described.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRON K. D., TUNCBAY T. O. PHOSPHATASE HISTOCHEMISTRY OF FELINE CERVICAL SPINAL CORD AFTER BRACHIAL PLEXECTOMY. HYDROLYSIS OF BETA-GLYCEROPHOSPHATE, THIAMINE PYROPHOSPHATE AND NUCLEOSIDE DIPHOSPHATES. J Neuropathol Exp Neurol. 1964 Apr;23:368–386. [PubMed] [Google Scholar]
- Barron K. D., Daniels A. C., Chiang T. Y., Doolin P. F. Fine structure of chromatolytic feline motoneurons. Exp Mol Pathol. 1970 Feb;12(1):46–57. doi: 10.1016/0014-4800(70)90074-2. [DOI] [PubMed] [Google Scholar]
- Burke B., Griffiths G., Reggio H., Louvard D., Warren G. A monoclonal antibody against a 135-K Golgi membrane protein. EMBO J. 1982;1(12):1621–1628. doi: 10.1002/j.1460-2075.1982.tb01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicheportiche Y., Vassalli P., Tartakoff A. M. Characterization of cytoplasmically oriented Golgi proteins with a monoclonal antibody. J Cell Biol. 1984 Dec;99(6):2200–2210. doi: 10.1083/jcb.99.6.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentinger M. P., Barron K. D., Kohberger R. C., McLean B. Cytologic observations on axotomized feline Betz cells. II. Quantitative ultrastructural findings. J Neuropathol Exp Neurol. 1979 Sep;38(5):551–564. doi: 10.1097/00005072-197909000-00008. [DOI] [PubMed] [Google Scholar]
- Gonatas J. O., Gonatas N. K., Stieber A., Fleischer B. Isolation and characterization of an enriched Golgi fraction from neurons of developing rat brains. J Neurochem. 1985 Aug;45(2):497–507. doi: 10.1111/j.1471-4159.1985.tb04016.x. [DOI] [PubMed] [Google Scholar]
- Gonatas J. O., Gonatas N. K., Stieber A., Louvard D. Polypeptides of the Golgi apparatus of neurons from rat brain. J Neurochem. 1987 Nov;49(5):1498–1506. doi: 10.1111/j.1471-4159.1987.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hoffman P. N., Cleveland D. W., Griffin J. W., Landes P. W., Cowan N. J., Price D. L. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci U S A. 1987 May;84(10):3472–3476. doi: 10.1073/pnas.84.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell K. E., Palade G. E. Hepatic Golgi fractions resolved into membrane and content subfractions. J Cell Biol. 1982 Mar;92(3):822–832. doi: 10.1083/jcb.92.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L. Protein A, avidin, and biotin in immunohistochemistry. J Histochem Cytochem. 1981 Nov;29(11):1349–1353. doi: 10.1177/29.11.6172466. [DOI] [PubMed] [Google Scholar]
- Lieberman A. R. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Louvard D., Reggio H., Warren G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J Cell Biol. 1982 Jan;92(1):92–107. doi: 10.1083/jcb.92.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten C., Stieber A., Grand E., Gonatas J., Gonatas N. K. Use of a minicomputer for quantitative ultrastructural autoradiography. J Histochem Cytochem. 1981 Apr;29(4):585–587. doi: 10.1177/29.4.7252126. [DOI] [PubMed] [Google Scholar]
- Perry G. W., Burmeister D. W., Grafstein B. Changes in protein content of goldfish optic nerve during degeneration and regeneration following nerve crush. J Neurochem. 1985 Apr;44(4):1142–1151. doi: 10.1111/j.1471-4159.1985.tb08736.x. [DOI] [PubMed] [Google Scholar]
- Price D. L., Porter K. R. The response of ventral horn neurons to axonal transection. J Cell Biol. 1972 Apr;53(1):24–37. doi: 10.1083/jcb.53.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. HISTOCHEMICAL AND ULTRASTRUCTURAL STUDIES ON HELA CELL CULTURES EXPOSED TO SPINDLE INHIBITORS WITH SPECIAL REFERENCE TO THE INTERPHASE CELL. J Histochem Cytochem. 1964 Sep;12:704–711. doi: 10.1177/12.9.704. [DOI] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. THE ULTRASTRUCTURE OF A MAMMALIAN CELL DURING THE MITOTIC CYCLE. J Cell Biol. 1964 Jun;21:429–463. doi: 10.1083/jcb.21.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski A. A., Singer S. J. Associations of elements of the Golgi apparatus with microtubules. J Cell Biol. 1984 Sep;99(3):1092–1100. doi: 10.1083/jcb.99.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A. H., Rohr H. P. Stereology, a complement to experimental neuropathology. I. Introduction into stereology. II. Ultrastructural-morphometric investigations (baseline data, axotomy) on the superior cervical ganglion of the rat. Acta Neuropathol. 1976 Oct 15;36(2):161–175. doi: 10.1007/BF00685278. [DOI] [PubMed] [Google Scholar]
- Stieber A., Gonatas J. O., Gonatas N. K., Louvard D. The Golgi apparatus-complex of neurons and astrocytes studied with an anti-organelle antibody. Brain Res. 1987 Apr 7;408(1-2):13–21. doi: 10.1016/0006-8993(87)90353-2. [DOI] [PubMed] [Google Scholar]
- Tougard C., Louvard D., Picart R., Tixier-Vidal A. Antibodies against a lysosomal membrane antigen recognize a prelysosomal compartment involved in the endocytic pathway in cultured prolactin cells. J Cell Biol. 1985 Mar;100(3):786–793. doi: 10.1083/jcb.100.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehland J., Willingham M. C. A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin. II. Effects on cell movement, organization of microtubules, and intermediate filaments, and arrangement of Golgi elements. J Cell Biol. 1983 Nov;97(5 Pt 1):1476–1490. doi: 10.1083/jcb.97.5.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]