Abstract
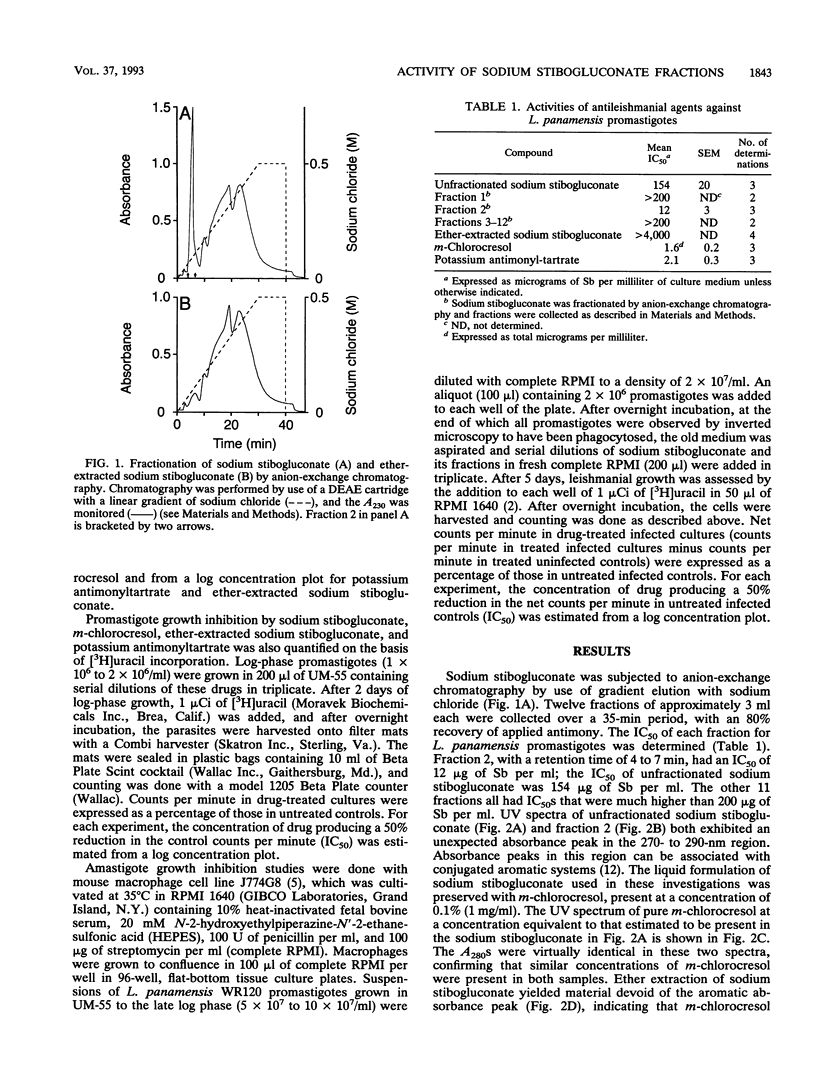
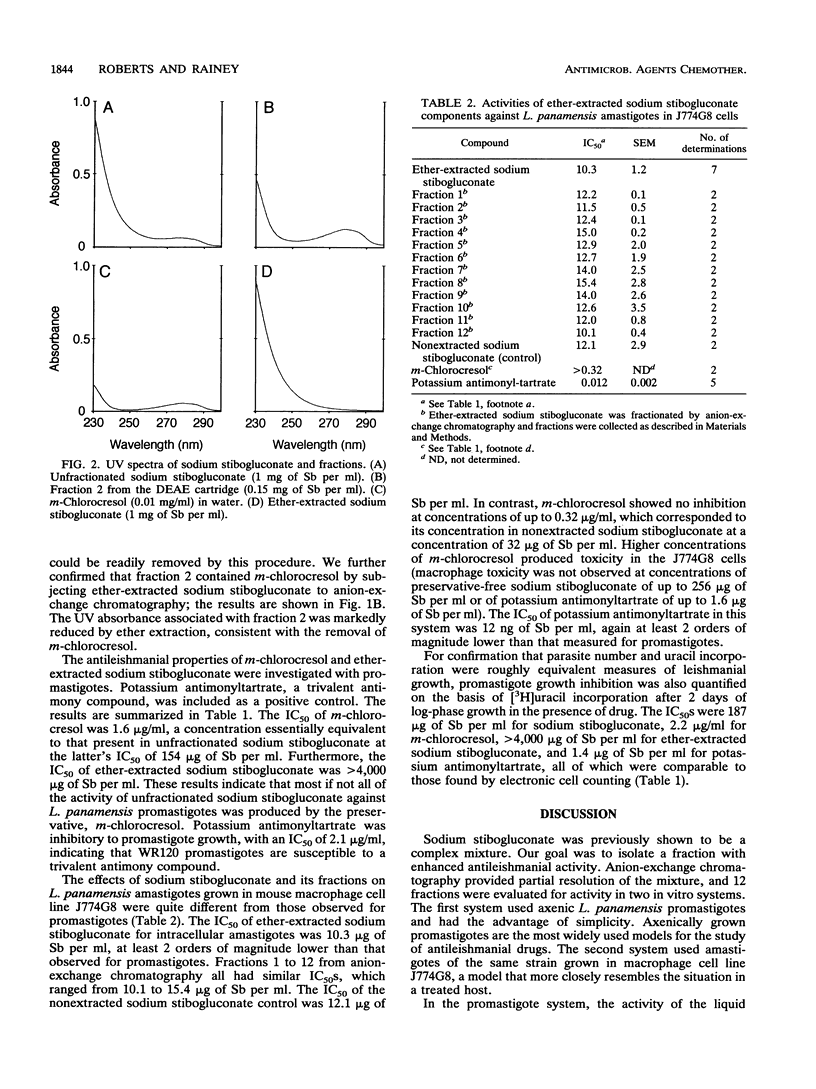
Sodium stibogluconate, a pentavalent antimony derivative produced by the reaction of stibonic and gluconic acids, is the drug of choice for the treatment of leishmaniasis. It has been reported to be a complex mixture rather than a single compound. We separated sodium stibogluconate into 12 fractions by anion-exchange chromatography. One fraction accounted for virtually all the leishmanicidal activity of the fractionated material against Leishmania panamensis promastigotes, with a 50% inhibitory concentration (IC50) of 12 micrograms of Sb per ml; that of unfractionated sodium stibogluconate was 154 micrograms of Sb per ml. Further analysis of this active fraction revealed that a major component was m-chlorocresol, which had been included in the sodium stibogluconate formulation as a preservative. The IC50 of pure m-chlorocresol was 1.6 micrograms/ml, a concentration equivalent to that present in unfractionated sodium stibogluconate at a concentration of 160 micrograms of Sb per ml. After ether extraction to remove m-chlorocresol, the IC50 of sodium stibogluconate was > 4,000 micrograms of Sb per ml. In contrast, when L. panamensis amastigotes were grown in macrophages, the IC50 of ether-extracted sodium stibogluconate was 10.3 micrograms of Sb per ml. The 12 fractions of ether-extracted sodium stibogluconate obtained by anion-exchange chromatography had IC50s of 10.1 to 15.4 micrograms of Sb per ml. We conclude that preservative-free sodium stibogluconate has little activity against L. panamensis promastigotes but is highly active against L. panamensis amastigotes in macrophages. This activity is associated with multiple chemical species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIGGS D. F. EXPERIMENTS ON THE MECHANISM OF ACTION OF CHLOROCRESOL AND CAFFEINE. Br J Pharmacol Chemother. 1965 Apr;24:510–518. doi: 10.1111/j.1476-5381.1965.tb01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaro R., Falcoff E., Badaro F. S., Carvalho E. M., Pedral-Sampaio D., Barral A., Carvalho J. S., Barral-Netto M., Brandely M., Silva L. Treatment of visceral leishmaniasis with pentavalent antimony and interferon gamma. N Engl J Med. 1990 Jan 4;322(1):16–21. doi: 10.1056/NEJM199001043220104. [DOI] [PubMed] [Google Scholar]
- Berman J. D., Gallalee J. V. Semiautomated assessment of in vitro activity of potential antileishmanial drugs. Antimicrob Agents Chemother. 1985 Dec;28(6):723–726. doi: 10.1128/aac.28.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. D., Grogl M. Leishmania mexicana: chemistry and biochemistry of sodium stibogluconate (Pentostam). Exp Parasitol. 1988 Oct;67(1):96–103. doi: 10.1016/0014-4894(88)90012-4. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Human cutaneous lieshmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980 Sep 12;209(4462):1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- Chulay J. D., Fleckenstein L., Smith D. H. Pharmacokinetics of antimony during treatment of visceral leishmaniasis with sodium stibogluconate or meglumine antimoniate. Trans R Soc Trop Med Hyg. 1988;82(1):69–72. [PubMed] [Google Scholar]
- Jackson J. E., Tally J. D., Ellis W. Y., Mebrahtu Y. B., Lawyer P. G., Were J. B., Reed S. G., Panisko D. M., Limmer B. L. Quantitative in vitro drug potency and drug susceptibility evaluation of Leishmania ssp. from patients unresponsive to pentavalent antimony therapy. Am J Trop Med Hyg. 1990 Nov;43(5):464–480. doi: 10.4269/ajtmh.1990.43.464. [DOI] [PubMed] [Google Scholar]
- Rainey P. M., Spithill T. W., McMahon-Pratt D., Pan A. A. Biochemical and molecular characterization of Leishmania pifanoi amastigotes in continuous axenic culture. Mol Biochem Parasitol. 1991 Nov;49(1):111–118. doi: 10.1016/0166-6851(91)90134-r. [DOI] [PubMed] [Google Scholar]
- Roberts W. L., Rainey P. M. Antimony quantification in Leishmania by electrothermal atomic absorption spectroscopy. Anal Biochem. 1993 May 15;211(1):1–6. doi: 10.1006/abio.1993.1223. [DOI] [PubMed] [Google Scholar]
- Steck E. A. The leishmaniases. Prog Drug Res. 1974;18:289–351. doi: 10.1007/978-3-0348-7087-0_22. [DOI] [PubMed] [Google Scholar]



