Abstract
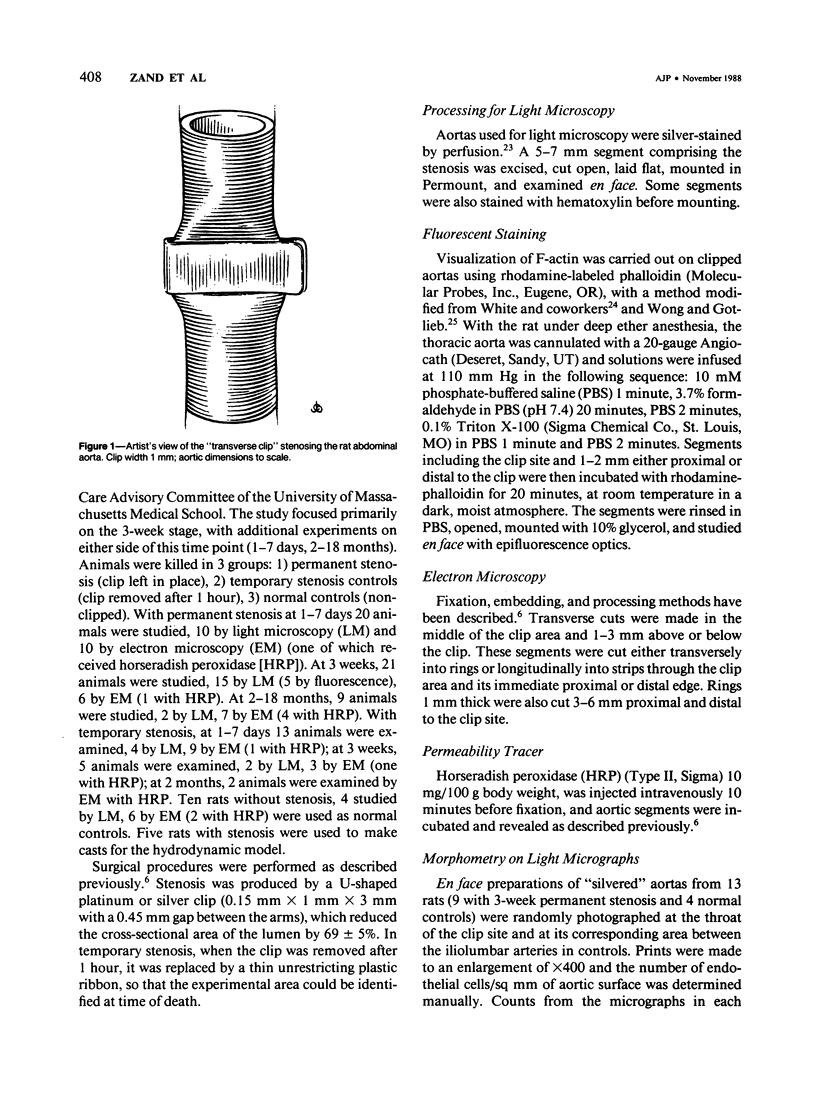
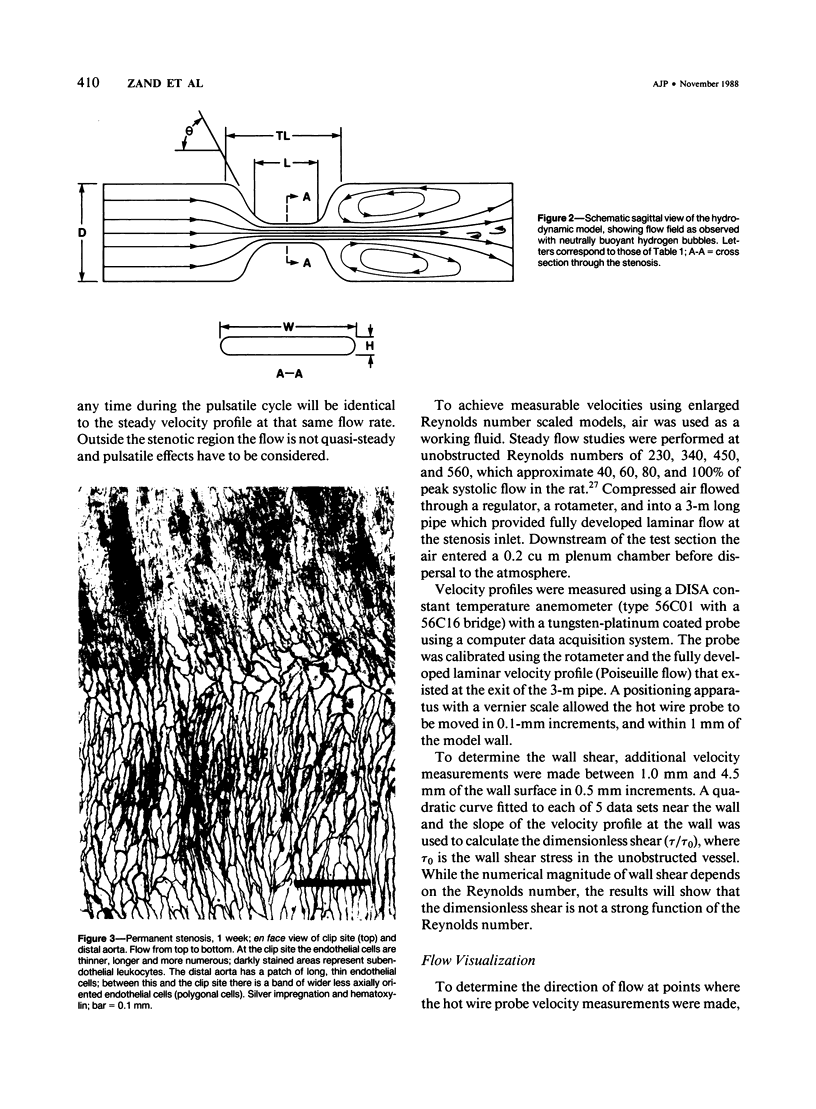
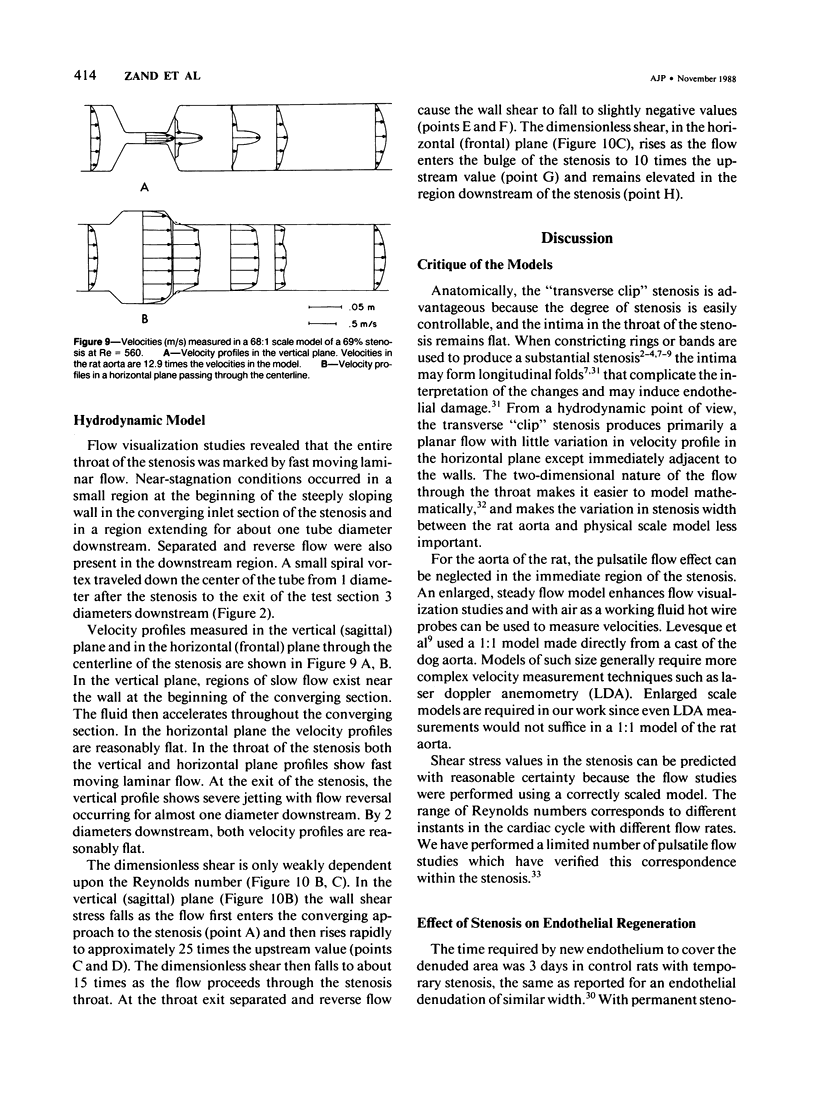
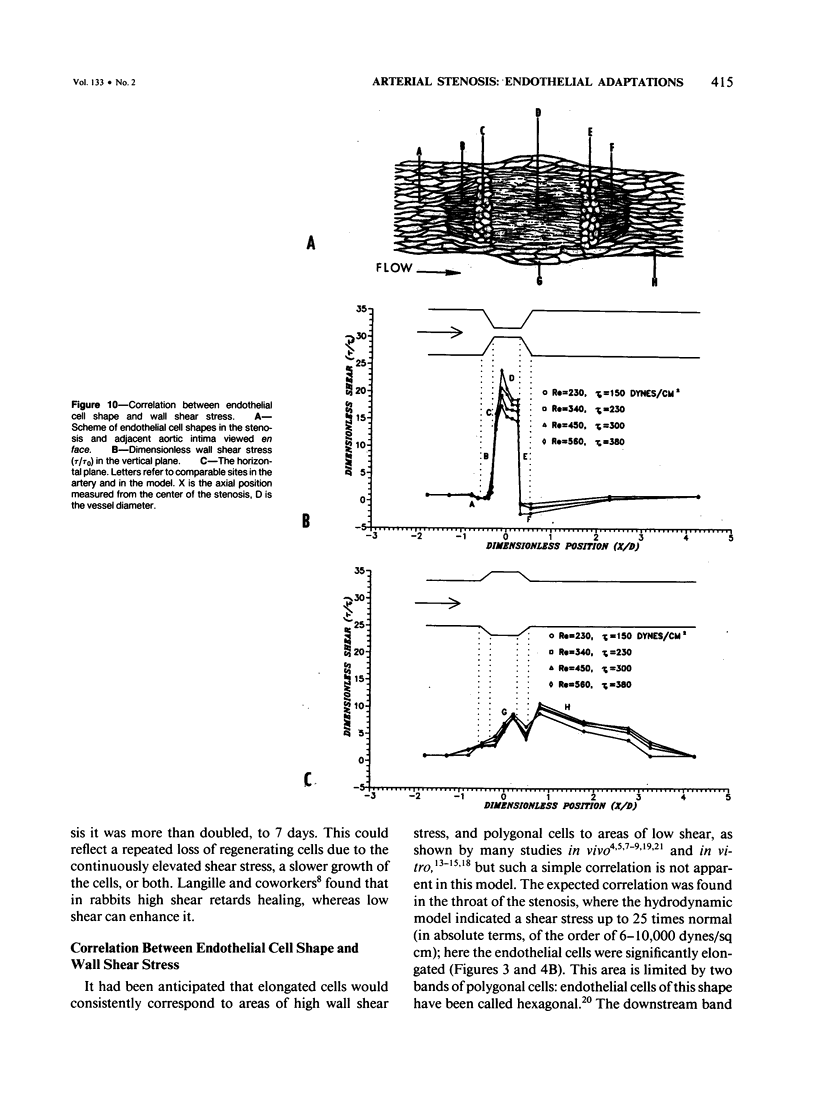
A 69 +/- 5% stenosis was produced in the rat aorta, with the purpose of correlating endothelial changes with local flow patterns and with levels of shear stress; the hydrodynamic data were obtained from a scaled-up model of the stenosed aorta. In the throat of the stenosis, where shear stress values were 15-25 times normal, the endothelium was stripped off within 1 hour. It regenerated at half the rate of controls but modulated into a cell type that could withstand the increased shear stress. Adaptations included changes in cell orientation, number, length, width, thickness, stress fibers, and anchoring structures, as well as changes in the length, argyrophilia, and permeability of the junctions. Areas of either elongated or "polygonal" cells consistently developed at the same sites in relation to the stenosis, but the hydrodynamic data showed that they did not always correspond (as had been anticipated) to high and low shear, respectively. It is concluded that endothelial cell shape in the living artery must be determined by some other factor(s) in addition to shear stress.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen W. T., Singer S. J. Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. J Cell Biol. 1982 Oct;95(1):205–222. doi: 10.1083/jcb.95.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. C., Chemnitz J., Tkocz I., Blaabjerg O. Repair in arterial tissue. The role of endothelium in the permeability of a healing intimal surface. Vital staining with Evans blue and silver-staining of the aortic intima after a single dilatation trauma. Acta Pathol Microbiol Scand A. 1977 May;85(3):297–310. [PubMed] [Google Scholar]
- Dewey C. F., Jr, Bussolari S. R., Gimbrone M. A., Jr, Davies P. F. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981 Aug;103(3):177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- Dewey C. F., Jr Effects of fluid flow on living vascular cells. J Biomech Eng. 1984 Feb;106(1):31–35. doi: 10.1115/1.3138453. [DOI] [PubMed] [Google Scholar]
- Eskin S. G., Ives C. L., McIntire L. V., Navarro L. T. Response of cultured endothelial cells to steady flow. Microvasc Res. 1984 Jul;28(1):87–94. doi: 10.1016/0026-2862(84)90031-1. [DOI] [PubMed] [Google Scholar]
- Franke R. P., Gräfe M., Schnittler H., Seiffge D., Mittermayer C., Drenckhahn D. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature. 1984 Feb 16;307(5952):648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- Fry D. L. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ Res. 1968 Feb;22(2):165–197. doi: 10.1161/01.res.22.2.165. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Gabbiani F., Lombardi D., Schwartz S. M. Organization of actin cytoskeleton in normal and regenerating arterial endothelial cells. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2361–2364. doi: 10.1073/pnas.80.8.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K. Alteration of endothelial cell surface morphology after experimental aortic coarctation. Artery. 1980;8(3):267–274. [PubMed] [Google Scholar]
- Glagov S., Ts'ao C. H. Restitution of aortic wall after sustained necrotizing transmural ligation injury. Role of blood cells and artery cells. Am J Pathol. 1975 Apr;79(1):7–30. [PMC free article] [PubMed] [Google Scholar]
- Gotlieb A. I., Spector W., Wong M. K., Lacey C. In vitro reendothelialization. Microfilament bundle reorganization in migrating porcine endothelial cells. Arteriosclerosis. 1984 Mar-Apr;4(2):91–96. doi: 10.1161/01.atv.4.2.91. [DOI] [PubMed] [Google Scholar]
- Greenhill N. S., Stehbens W. E. Scanning electron-microscopic study of the anastomosed vein of arteriovenous fistulae. Atherosclerosis. 1981 Jun;39(3):383–393. doi: 10.1016/0021-9150(81)90024-1. [DOI] [PubMed] [Google Scholar]
- Haudenschild C. C., Schwartz S. M. Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest. 1979 Nov;41(5):407–418. [PubMed] [Google Scholar]
- Herman I. M., Pollard T. D., Wong A. J. Contractile proteins in endothelial cells. Ann N Y Acad Sci. 1982;401:50–60. doi: 10.1111/j.1749-6632.1982.tb25706.x. [DOI] [PubMed] [Google Scholar]
- Hüttner I., Walker C., Gabbiani G. Aortic endothelial cell during regeneration. Remodeling of cell junctions, stress fibers, and stress fiber-membrane attachment domains. Lab Invest. 1985 Sep;53(3):287–302. [PubMed] [Google Scholar]
- Ives C. L., Eskin S. G., McIntire L. V. Mechanical effects on endothelial cell morphology: in vitro assessment. In Vitro Cell Dev Biol. 1986 Sep;22(9):500–507. doi: 10.1007/BF02621134. [DOI] [PubMed] [Google Scholar]
- Joris I., Majno G. Endothelial changes induced by arterial spasm. Am J Pathol. 1981 Mar;102(3):346–358. [PMC free article] [PubMed] [Google Scholar]
- Joris I., Zand T., Majno G. Hydrodynamic injury of the endothelium in acute aortic stenosis. Am J Pathol. 1982 Mar;106(3):394–408. [PMC free article] [PubMed] [Google Scholar]
- Kibria G., Heath D., Smith P., Biggar R. Pulmonary endothelial pavement patterns. Thorax. 1980 Mar;35(3):186–191. doi: 10.1136/thx.35.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille B. L., Reidy M. A., Kline R. L. Injury and repair of endothelium at sites of flow disturbances near abdominal aortic coarctations in rabbits. Arteriosclerosis. 1986 Mar-Apr;6(2):146–154. doi: 10.1161/01.atv.6.2.146. [DOI] [PubMed] [Google Scholar]
- Legg M. J., Gow B. S. Scanning electron microscopy of endothelium around an experimental stenosis in the rabbit aorta using a new casting material. Atherosclerosis. 1982 Apr;42(2-3):299–318. doi: 10.1016/0021-9150(82)90158-7. [DOI] [PubMed] [Google Scholar]
- Levesque M. J., Cornhill J. F., Nerem R. M. Vascular casting. A new method for the study of the arterial endothelium. Atherosclerosis. 1979 Dec;34(4):457–467. doi: 10.1016/0021-9150(79)90070-4. [DOI] [PubMed] [Google Scholar]
- Levesque M. J., Liepsch D., Moravec S., Nerem R. M. Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta. Arteriosclerosis. 1986 Mar-Apr;6(2):220–229. doi: 10.1161/01.atv.6.2.220. [DOI] [PubMed] [Google Scholar]
- Levesque M. J., Nerem R. M. The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng. 1985 Nov;107(4):341–347. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Langille B. L. The effect of local blood flow patterns on endothelial cell morphology. Exp Mol Pathol. 1980 Jun;32(3):276–289. doi: 10.1016/0014-4800(80)90061-1. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Reidy M. A., Silver M. Endothelial regeneration. VII. Lack of intimal proliferation after defined injury to rat aorta. Am J Pathol. 1985 Feb;118(2):173–177. [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Cliff W. J., Gabbiani G., Irlé C., Montandon D., Statkov P. R., Majno G. Myofibroblasts in human granulation tissue. Hum Pathol. 1974 Jan;5(1):55–67. doi: 10.1016/s0046-8177(74)80100-0. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Kazazis D. M., Clark R. A. In vivo co-distribution of fibronectin and actin fibers in granulation tissue: immunofluorescence and electron microscope studies of the fibronexus at the myofibroblast surface. J Cell Biol. 1984 Jun;98(6):2091–2106. doi: 10.1083/jcb.98.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Kazazis D. M., Kawka D. W. Localization of the fibronexus at the surface of granulation tissue myofibroblasts using double-label immunogold electron microscopy on ultrathin frozen sections. Eur J Cell Biol. 1985 Jul;38(1):94–101. [PubMed] [Google Scholar]
- Viggers R. F., Wechezak A. R., Sauvage L. R. An apparatus to study the response of cultured endothelium to shear stress. J Biomech Eng. 1986 Nov;108(4):332–337. doi: 10.1115/1.3138624. [DOI] [PubMed] [Google Scholar]
- Wechezak A. R., Viggers R. F., Sauvage L. R. Fibronectin and F-actin redistribution in cultured endothelial cells exposed to shear stress. Lab Invest. 1985 Dec;53(6):639–647. [PubMed] [Google Scholar]
- White G. E., Gimbrone M. A., Jr, Fujiwara K. Factors influencing the expression of stress fibers in vascular endothelial cells in situ. J Cell Biol. 1983 Aug;97(2):416–424. doi: 10.1083/jcb.97.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. K., Gotlieb A. I. In vitro reendothelialization of a single-cell wound. Role of microfilament bundles in rapid lamellipodia-mediated wound closure. Lab Invest. 1984 Jul;51(1):75–81. [PubMed] [Google Scholar]
- Zand T., Underwood J. M., Nunnari J. J., Majno G., Joris I. Endothelium and "silver lines". An electron microscopic study. Virchows Arch A Pathol Anat Histol. 1982;395(2):133–144. doi: 10.1007/BF00429607. [DOI] [PubMed] [Google Scholar]








