Abstract
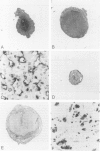
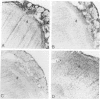
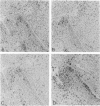
Two different hormonal regimens to induce pseudopregnancy resulted in a pronounced increase in the susceptibility of the murine uterus to intraluminal injections of Listeria monocytogenes. Preimmunization, which profoundly augments systemic listeria resistance, had no effect on this increased uterine susceptibility. Anti-listerial responses in other organs were unaffected by pseudopregnancy. Animals manifesting increased susceptibility formed distinct uterine swellings in response to the combination of hormones and uterine listeria. These swellings correspond to previously described deciduoma and closely mimic the decidualized endometrium of pregnancy. The nature of the defective response to listeria was investigated by immunocytochemistry. Increased bacterial titers were correlated with an inability of macrophages and T lymphocytes to reach tissue listeria in discrete regions of deciduoma-bearing uteri. Control uteri showed a normal granulomatous pattern of inflammation. These findings closely parallel previous findings in the murine decidua basalis and suggest that properties of decidualized endometrial stromal cells regulate local immune responsiveness.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Badet M. T., Bell S. C., Billington W. D. Immunoregulatory activity of supernatants from short-term cultures of mouse decidual tissue. J Reprod Fertil. 1983 Jul;68(2):351–358. doi: 10.1530/jrf.0.0680351. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Finn C. A., Martin L. Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod. 1972 Aug;7(1):82–86. doi: 10.1093/biolreprod/7.1.82. [DOI] [PubMed] [Google Scholar]
- Finn C. A. The biology of decidual cells. Adv Reprod Physiol. 1971;5:1–26. [PubMed] [Google Scholar]
- Gibori G., Kalison B., Basuray R., Rao M. C., Hunzicker-Dunn M. Endocrine role of the decidual tissue: decidual luteotropin regulation of luteal adenylyl cyclase activity, luteinizing hormone receptors, and steroidogenesis. Endocrinology. 1984 Sep;115(3):1157–1163. doi: 10.1210/endo-115-3-1157. [DOI] [PubMed] [Google Scholar]
- Hunziker R. D., Wegmann T. G. Placental immunoregulation. Crit Rev Immunol. 1986;6(3):245–285. [PubMed] [Google Scholar]
- Leavitt W. W., Takeda A. Hormonal regulation of estrogen and progestin receptors in decidual cells. Biol Reprod. 1986 Sep;35(2):475–484. doi: 10.1095/biolreprod35.2.475. [DOI] [PubMed] [Google Scholar]
- Lepay D. A., Steinman R. M., Nathan C. F., Murray H. W., Cohn Z. A. Liver macrophages in murine listeriosis. Cell-mediated immunity is correlated with an influx of macrophages capable of generating reactive oxygen intermediates. J Exp Med. 1985 Jun 1;161(6):1503–1512. doi: 10.1084/jem.161.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn D. M., Bargatze R. F., Butcher E. C. Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol. 1987 Jun 15;138(12):4313–4321. [PubMed] [Google Scholar]
- Mason D. W., Morris P. J. Effector mechanisms in allograft rejection. Annu Rev Immunol. 1986;4:119–145. doi: 10.1146/annurev.iy.04.040186.001003. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- Nose A., Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986 Dec;103(6 Pt 2):2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näher H., Sperling U., Takacs L., Hahn H. Dynamics of T cells of L3T4 and Ly 2 phenotype within granulomas in murine listeriosis. Clin Exp Immunol. 1985 Jun;60(3):559–564. [PMC free article] [PubMed] [Google Scholar]
- O'Shea J. D., Kleinfeld R. G., Morrow H. A. Ultrastructure of decidualization in the pseudopregnant rat. Am J Anat. 1983 Mar;166(3):271–298. doi: 10.1002/aja.1001660304. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Peel S., Stewart I. Oestrogen and the differentiation of granulated metrial gland cells in chimeric mice. J Anat. 1986 Feb;144:181–187. [PMC free article] [PubMed] [Google Scholar]
- Pollard J. W., Bartocci A., Arceci R., Orlofsky A., Ladner M. B., Stanley E. R. Apparent role of the macrophage growth factor, CSF-1, in placental development. Nature. 1987 Dec 3;330(6147):484–486. doi: 10.1038/330484a0. [DOI] [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Role of local immunosuppression in murine fetoplacental listeriosis. J Clin Invest. 1987 Apr;79(4):1234–1241. doi: 10.1172/JCI112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J Immunol. 1988 Jun 1;140(11):3947–3955. [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Poole A. R., Lewandowska K., Culp L. A. Biological roles of dermatan sulphate proteoglycans. Ciba Found Symp. 1986;124:47–68. doi: 10.1002/9780470513385.ch4. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Sharma R., Bulmer D., Peel S. Effects of exogenous progesterone following ovariectomy on the metrial glands of pregnant mice. J Anat. 1986 Feb;144:189–199. [PMC free article] [PubMed] [Google Scholar]
- Stites D. P., Siiteri P. K. Steroids as immunosuppressants in pregnancy. Immunol Rev. 1983;75:117–138. doi: 10.1111/j.1600-065x.1983.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Tachi C., Tachi S. Macrophages and implantation. Ann N Y Acad Sci. 1986;476:158–182. doi: 10.1111/j.1749-6632.1986.tb20929.x. [DOI] [PubMed] [Google Scholar]
- Tung H. N., Parr M. B., Parr E. L. The permeability of the primary decidual zone in the rat uterus: an ultrastructural tracer and freeze-fracture study. Biol Reprod. 1986 Nov;35(4):1045–1058. doi: 10.1095/biolreprod35.4.1045. [DOI] [PubMed] [Google Scholar]
- Welsh A. O., Enders A. C. Light and electron microscopic examination of the mature decidual cells of the rat with emphasis on the antimesometrial decidua and its degeneration. Am J Anat. 1985 Jan;172(1):1–29. doi: 10.1002/aja.1001720102. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Damjanov A., Weiss J., Liotta L. A., Damjanov I. Mouse endometrial stromal cells produce basement-membrane components. Differentiation. 1986;32(1):49–58. doi: 10.1111/j.1432-0436.1986.tb00555.x. [DOI] [PubMed] [Google Scholar]
- Zardi L., Carnemolla B., Siri A., Petersen T. E., Paolella G., Sebastio G., Baralle F. E. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J. 1987 Aug;6(8):2337–2342. doi: 10.1002/j.1460-2075.1987.tb02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]