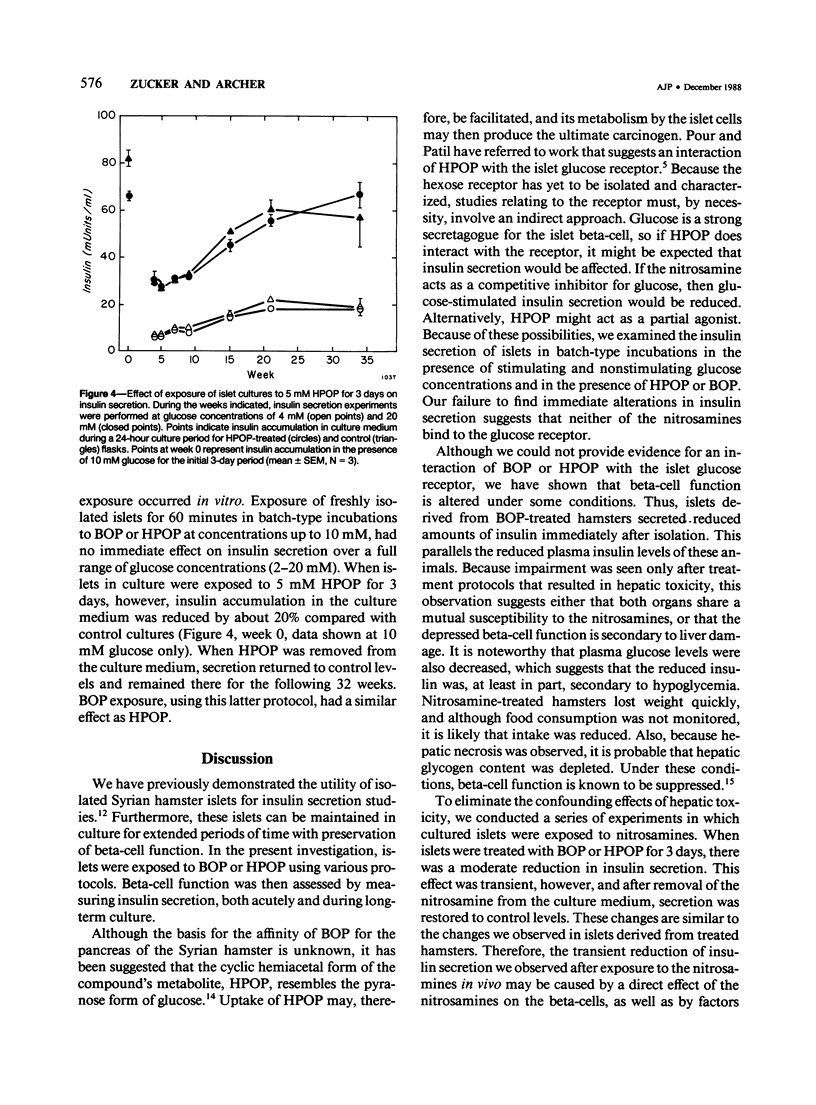
Abstract
Exposure of hamsters to 5 daily doses of 20 mg/kg N-nitrosobis(2-oxopropyl)amine (BOP) or 76 mg/kg N-nitroso(2-hydroxypropyl)(2-oxopropyl)amine (HPOP), resulted in reduced insulin secretion in freshly isolated pancreatic islets. These treatments also reduced plasma insulin and glucose levels, and were hepatotoxic. The inhibition of insulin secretion, however, was transient. Islets isolated from treated hamsters that were then placed in culture secreted elevated levels of insulin for many months. When cultured islets were directly exposed to the nitrosamines for 3 days, there was also a transient reduction of insulin secretion that was subsequently normalized after removal of the nitrosamine from the medium. These results show that BOP and HPOP modify beta-cell function both directly, and possibly indirectly, via damage to the liver. Furthermore, the lack of immediate inhibition of insulin secretion when islets were incubated in the presence of BOP or HPOP as well as glucose, suggests that the nitrosamines do not bind to the glucose receptor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. H., Jr, Strayer D. S. Streptozotocin prevents development of nitrosamine-induced pancreatic cancer in the Syrian hamster. J Surg Oncol. 1983 Dec;24(4):258–262. doi: 10.1002/jso.2930240404. [DOI] [PubMed] [Google Scholar]
- Boux L. J., Leung K. H., Sweet M. E., Archer M. C. Enzymatic reduction of beta-ketonitrosamines. Carcinogenesis. 1983 Nov;4(11):1495–1498. doi: 10.1093/carcin/4.11.1495. [DOI] [PubMed] [Google Scholar]
- Doria J. C., Mosley J. G., Reber H. A. Effect of a single exposure to a carcinogen on pancreatic function in hamsters. J Surg Res. 1981 Feb;30(1):21–26. doi: 10.1016/0022-4804(81)90066-4. [DOI] [PubMed] [Google Scholar]
- Jain K., Zucker P. F., Chan A. M., Archer M. C. Monolayer culture of pancreatic islets from the Syrian hamster. In Vitro Cell Dev Biol. 1985 Jan;21(1):1–5. doi: 10.1007/BF02620906. [DOI] [PubMed] [Google Scholar]
- Pour P. M., Donnelly K., Stepan K. Modification of pancreatic carcinogenesis in the hamster model. 3. Inhibitory effect of alloxan. Am J Pathol. 1983 Mar;110(3):310–314. [PMC free article] [PubMed] [Google Scholar]
- Pour P. M., Patil K. Modification of pancreatic carcinogenesis in the hamster model. X. Effect of streptozotocin. J Natl Cancer Inst. 1983 Nov;71(5):1059–1065. [PubMed] [Google Scholar]
- Pour P. M., Raha C. R. Pancreatic carcinogenic effect of N-nitrosobis (2-oxobutyl) amine and N-nitroso (2-oxobutyl) (2-oxopropyl) amine in Syrian hamster. Cancer Lett. 1981 Apr;12(3):223–229. doi: 10.1016/0304-3835(81)90072-0. [DOI] [PubMed] [Google Scholar]
- Pour P. M., Stepan K. Modification of pancreatic carcinogenesis in the hamster model. VIII. Inhibitory effect of exogenous insulin. J Natl Cancer Inst. 1984 May;72(5):1205–1208. [PubMed] [Google Scholar]
- Pour P. M., Wallcave L., Nagel D. The effect of N-nitroso-2-methoxy-2,6-dimethylmorpholine on endocrine and exocrine pancreas of Syrian hamsters. Cancer Lett. 1981 Aug;13(3):233–240. doi: 10.1016/0304-3835(81)90023-9. [DOI] [PubMed] [Google Scholar]
- Pour P., Althoff J., Krüger F., Schmähl D., Mohr U. Induction of pancreatic neoplasms by 2,2'-dioxopropyl-N-propylnitrosamine. Cancer Lett. 1975 Sep;1(1):3–6. doi: 10.1016/s0304-3835(75)94491-2. [DOI] [PubMed] [Google Scholar]
- Pour P. Islet cells as a component of pancreatic ductal neoplasms. I. Experimental study: ductular cells, including islet cell precursors, as primary progenitor cells of tumors. Am J Pathol. 1978 Feb;90(2):295–316. [PMC free article] [PubMed] [Google Scholar]
- Pour P., Wallcave L., Gingell R., Nagel D., Lawson T., Salmasi S., Tines S. Carcinogenic effect of N-nitroso(2-hydroxypropyl)(2-oxopropyl)amine, a postulated proximate pancreatic carcinogen in Syrian hamsters. Cancer Res. 1979 Oct;39(10):3828–3833. [PubMed] [Google Scholar]
- Zucker P., Logothetopoulos J. Persisting enhanced proinsulin-insulin and protein biosynthesis (3H-leucine incorporation) by pancreatic islets of the rat after glucose exposure. Diabetes. 1975 Feb;24(2):194–200. doi: 10.2337/diab.24.2.194. [DOI] [PubMed] [Google Scholar]


