Summary
Background
The small GTPase Rac1 plays a critical role in lamellipodia assembly in platelets on matrix proteins in the absence or presence of G protein-coupled receptor (GPCR) agonists. Rac mediates actin assembly via Scar/WAVE, a family of scaffolding proteins that direct actin reorganization by relaying signals from Rac to the Arp2/3 complex.
Objective
To evaluate the role of Scar/WAVE-1 in mediating platelet activation and cytoskeletal reorganisation.
Methods and Results
Using specific antibodies, we demonstrate that murine platelets, like human platelets, express Scar/WAVE-1 and Scar/WAVE-2. Lamellipodia formation in Scar/WAVE-1-/- platelets is markedly inhibited on immobilized CRP and on laminin, both of which signal through the collagen receptor GPVI. In contrast, lamellipodia formation on collagen, which requires release of the GPCR agonists ADP and thromboxane A2 is not altered. Immobilized fibrinogen supports limited formation of lamellipodia in murine platelets which is not altered in Scar/WAVE-1-/- platelets. As with Rac1-/- platelets, Scar/WAVE-1-/- platelets, exhibit a marked inhibition of aggregation in response to CRP whereas the response to the GPCR agonist thrombin is not altered. Platelet aggregation on immobilized collagen under shear, which is dependent on signalling by matrix and GPCR agonists, was unaltered in the absence of Scar/WAVE-1.
Conclusion
This study demonstrates a major role for Scar/WAVE-1 in mediating platelet cytoskeletal reorganization and aggregate formation downstream of activation by GPVI but not by GPCR agonists.
Keywords: Scar/WAVE-1, platelets, cytoskeletal reorganization
INTRODUCTION
Actin assembly is important in mediating changes in platelet morphology during thrombus formation. The amount of actin assembled in activated platelets approximately doubles as platelets change from an inactive discoid shape to a sphere and then form spiky filopodia that progress to lamellipodia [1-4]. During this process, multiple receptor-mediated signalling cascades regulate rapid remodelling of the platelet cytoskeleton.
It has long been proposed that the Rho-family small GTPases Rho, Rac and Cdc42 are likely to be important in reorganization of the actin cytoskeleton of the platelet [5, 6]. We recently found that Rac1 is essential for lamellipodia formation in platelets following adhesion and activation on fibrinogen (in the presence of thrombin), collagen and laminin, but that it is not required for the doubling of F-actin in response to thrombin [3]. Importantly, the loss of thrombus stability observed under shear conditions in vitro and in vivo in the absence of Rac1 [3] demonstrates the essential involvement of platelet cytoskeletal regulation in the process of thrombus formation.
Scar/WAVE (Scar) proteins are major Rac targets that mediate actin assembly into lamellipodia via the Arp2/3 complex [7-10]. Scar/WAVE-1 and -2 are believed to be the major two isoforms of Scar/WAVE in human platelets, although the presence of a low level of Scar/WAVE-3 has also been reported [11, 12]. The three Scar/WAVE isoforms have many redundant features [13,14] and thus might compensate for each other.
In the present study, we have identified that murine platelets express Scar/WAVE-1 and Scar/WAVE-2. We have characterized platelets from a Scar/WAVE-1 knock-out mouse for their ability to adhere to various matrices in the absence and presence of GPCR agonists. This study demonstrates that GPVI signalling is heavily compromised by the removal of Scar/WAVE-1, as evidenced by the almost complete blockage of aggregation and severely delayed shape change to sub-maximal concentrations of the specific GPVI ligand, collagen-related peptide (CRP). Moreover, lamellipodia formation in response to CRP and to laminin, which also signals via GPVI [15], is abrogated in the absence of Scar/WAVE-1, although it is restored in the presence of thrombin or ADP. On the other hand, Scar/WAVE-1 does not play a critical role in mediating aggregation or lamellipodia formation on fibrinogen induced by the G protein-coupled receptor (GPCR) agonist thrombin. Taken together, this study implies a major role for Scar/WAVE-1 downstream of GPVI.
EXPERIMENTAL PROCEDURES
Reagents
The anti-Scar/WAVE-2 polyclonal antibody was purchased from Upstate Biochemical (Dundee, UK). Anti-Scar/WAVE-1 antibody was made as described [14]. FITC-linked P-selectin antibody was purchased from BD Pharminogen (Oxford, UK). All other reagents were from Sigma (Poole, UK) or previously named sources [3, 17]. Scar/WAVE-1 and Scar/WAVE-2-expression plasmids were constructed as previously described [14]. Recombinant plasmids were transfected into Cos-7 cells using lipofectamine (Gibco, Paisley, UK).
Scar/WAVE-1-/- mice were a kind gift of Seung Kwak at Wyeth Research, Princeton, NJ. USA [18]. Mice were bred as heterozygotes and all experiments were performed on mice aged 14-20 days of age using litter-matched controls. The mice were used at this age, as the absence of Scar/WAVE-1 leads to death at later times [18]. Knockout mice were identified by the small phenotype and later genotyped as previously described [14].
Preparation of murine and human washed platelets
Murine blood was drawn from CO2 terminally narcosed mice under anaesthetic from the hepatic portal vein and taken into ACD at a ratio of 1:10. Haematological parameters of whole murine blood were determined using an ABX Micros 60 (ABX Diagnostics, Montpelier, France). Washed murine platelets were prepared as previously described [3, 17]. Platelets were resuspended in modified HEPES-Tyrode buffer (in mM: 129 NaCl, 0.34 Na2HPO4, 2.9 KCl, 12 NaHCO3, 20 HEPES, 5 glucose, 1 MgCl2; pH 7.3) to the desired platelet level. All animals were maintained using housing and husbandry in accordance with local and national legal regulations. Human washed platelets were prepared as previously described [17].
Static adhesion assays
Cover slips were incubated with a suspension of fibrinogen (100 μg/ml), collagen (100 μg/ml), laminin (50 μg/ml) or CRP (100 μg/ml) overnight at 4°C. Surfaces were then blocked with denatured BSA (5 mg/ml) for 1 hour at room temperature followed by subsequent washing with PBS before use in spreading assays. Platelets (2 × 107/ml) were layered on immobilized proteins and allowed to adhere for 45 minutes at 37°C. Surfaces were then washed with PBS to remove non-adherent cells before fixation with 10% formalin, neutral buffered, for 10 minutes at room temperature. Only an occasional platelet bound to surfaces coated with denatured BSA (data not shown). Platelet morphology was imaged using Köhler illuminated Nomarski differential interference contrast optics with a Zeiss 63× oil immersion 1.40 NA plan-apochromat lens on a Zeiss Axiovert 200M Microscope. Digital images were captured by a Hamamatsu Orca 285 cooled digital camera (Cairn Research, Kent, UK) using Slidebook 4.0 (Intelligent Imaging Innovations, Inc., Denver, CO, USA). The degree of platelet adhesion and surface area of spread platelet were computed using a java plugin for the Image J software package as previously described [17].
Measurement of P-selectin expression by flow cytometry
Washed platelets (2 × 107/ml), in the presence of 2 mM Ca2+, were treated with either thrombin (0-0.1 U/ml), collagen (0-30 μg/ml) or the GPVI-specific agonist CRP (0-30 μg/ml) in the presence of a FITC-conjugated anti-CD62P mAb (10 μg/ml) for 15 min at 37°C. In selected experiments, fluorescently-labelled isotype-matched IgG mAbs were included for background fluorescence determination. Samples were diluted with PBS, followed by flow cytometric analysis on a Beckman Dickinson FACScalibur. Platelets were identified by logarithmic signal amplification for forward and side scatter, and the geometric mean fluorescence of each specimen was recorded.
Platelet aggregation and flow adhesion studies
Platelet aggregation was monitored using either 250 μl of washed platelets (2 × 108/ml) or 50μl (1.2×108/ml). Stimulation of platelets (2×108/ml) was performed in a Chrono-Log aggregometer (Chrono-Log, Havertown, PA, USA) with continuous stirring at 1200 rpm at 37°C as previously described [3]. Pre/post platelet counts (1.2×108/ml) were performed using Coulter counter (Becton Dickinson, USA). Post platelet counts were taken at two minutes after stimulation.
For flow adhesion studies, mouse blood was drawn into sodium heparin (10 IU/ml) and PPACK (40 μM). Glass capillary tubes (Camlab, Cambridge, UK) were coated with 100 μg/ml type I collagen from equine tendon (Horm, Nycomed, Munich, Germany) for 1 h at room temperature (22 ± 2 °C). The capillaries were washed and blocked with PBS containing 5 mg/ml BSA for 1 h at room temperature before being mounted on the stage of an inverted microscope (DM IRB; Leica, Milton Keynes, UK). Anticoagulated whole blood was perfused through the chamber for 4 min at a wall shear rate of 1000 s-1, followed by washing for 3 min at the same shear rate with modified Tyrodes buffer before imaged using phase-contrast microscopy. Image analysis was performed off-line using ImageJ. Platelet adhesion results are expressed as the percentage of surface area covered by platelets.
Analysis of data
Results shown are representative of data from one experiment. Where applicable, results are shown as mean ± SEM. Statistical significance of differences between means was determined by ANOVA. If means were shown to be significantly different, multiple comparisons were performed by the Tukey test. Probability values of P < 0.05 were selected to be statistically significant.
RESULTS
Identification of Scar/WAVE isoforms present in platelets
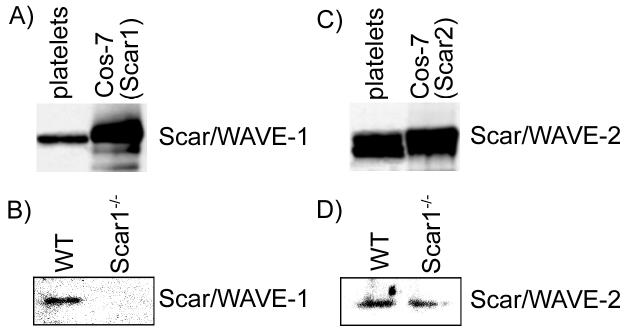
To study the role of Scar/WAVE proteins in regulating the organization of the platelet actin cytoskeleton, we first examined which Scar/WAVE isoforms are expressed in mouse platelets alongside studies in human platelets. Platelet lysates were processed for Western blot analysis with isoform specific Scar/WAVE antibodies. Immunoblot analyses established that both human and mouse platelets express Scar/WAVE-1 and Scar/WAVE-2 (Fig. 1). We were unable to detect Scar/WAVE-3 in human or mouse platelets using available antibodies (data not shown). Scar/WAVE-1-/- platelets did not express Scar/WAVE-1 (Fig. 1B), as anticipated, whereas the level of Scar/WAVE-2 was not altered (Fig. 1D). It is noteworthy that leukocyte production is reduced, but red blood cell and platelet production and average platelet volume are maintained in Scar/WAVE-1-deficient mice as compared with the levels observed in wild-type mice (Table 1). This suggests that while Scar/WAVE-1 may play a role in leukocyte production, Scar/WAVE-1 does not play a role in platelet production.
Fig. 1.
Scar/WAVE isoforms are expressed in human and murine platelets. Equal amounts of human (A and C) and murine wild-type and Scar/WAVE-1-/- (B and D) platelet lysates were analyzed for Scar/WAVE expression using Scar/WAVE-1 or Scar/WAVE-2 antibodies. Lysates from Cos-7 cells transfected with a vector containing (A) Scar/WAVE-1 or (C) Scar/WAVE-2 were included as a positive control.
Table 1.
Haematological parameters of wild-type and Scar/WAVE-1-/- mice.
| Parameter | Wild-type | Scar/WAVE-1-/- |
|---|---|---|
| Leukocytes, 103/ml | 3.39 ± 0.67 | 1.09 ± 0.21* |
| Erythrocytes, 106/ml | 3.54 ± 0.14 | 3.39 ± 0.57 |
| Platelets, 106/ml | 329.2 ± 18.9 | 260.4 ± 47.8 |
| Platelet volume, fL | 5.24 ± 0.07 | 5.28 ± 0.16 |
Samples of whole blood (50 μl) were analyzed using an ABX Micros 60. Data is presented as mean ± SD from 5 mice.
P < 0.01 with respect to wild-type.
Scar/WAVE-1 is required for lamellipodia formation on CRP and laminin
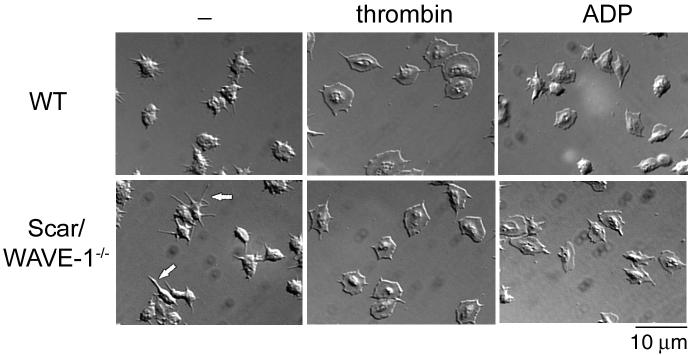
We assayed the ability of Scar/WAVE-1-deficient platelets to adhere and spread on a number of immobilized ligands in the absence and presence of GPCR agonists. As shown in Fig. 2, wild-type platelets generate filopodia upon adhesion to a fibrinogen surface and lose the characteristic biconcave disc shape of resting cells. Further, wild type platelets generate circumferential lamellae in the presence of the GPCR agonists, thrombin and ADP, with a more marked response seen in the presence of the more powerful agonist thrombin. There was no significant alteration in the degree of ‘spreading’ of Scar/WAVE-1-/- murine platelets relative to their wild type controls under any of the above conditions as determined by measurement of platelet surface area (Table 2). On the other hand, 26.2 ± 0.3% of the Scar/WAVE-1-/- platelets produced elongated filopodia in the absence of thrombin and ADP that were at least twice the length of those seen in wild-type platelets (see arrow in Fig. 2). Interestingly, prolonged filopodia (and absence of lamellipodia) are also seen on fibrinogen in Rac1-/- mice in the presence of the GPCR agonist thrombin [3], and presumably reflect the assembly of actin into unbranched filaments rather than branching into lamellipodia. The elongated filopodia seen in the absence of Scar/WAVE may represent a similar event. Overall the results indicate a minor role for Scar/WAVE-1 in actin assembly on fibrinogen in the absence of G protein receptor agonists and no role in their presence.
Fig. 2.
Role of Scar/WAVE-1 in αIIbβ3-mediated murine platelet spreading. Purified wild-type (WT) and Scar/WAVE-1-/- murine platelets (2 × 107/ml) were placed on coverslips coated with fibrinogen with or without 1 U/ml thrombin for 45 min and imaged using DIC microscopy. In separate experiments, platelets were treated with 10 μM ADP. Note the elongated filopodia extended by a portion of the Scar/WAVE-1-/- platelets on fibrinogen under non-stimulated conditions as indicated by the arrows. Results are representative of at least 3 experiments.
Table 2.
Platelet surface area in wild-type and Scar/WAVE-1-/- mice following adhesion.
| Murine Platelet Spreading (SA; μm2) | |||
|---|---|---|---|
| Surface | Treatment | WT | Scar/WAVE-1-/- |
| Fibrinogen | - | 14.7 ± 0.12 | 14.2 ± 0.28 |
| Fibrinogen | ADP | 18.4 ± 0.18 | 18.1 ± 0.16 |
| Fibrinogen | thrombin | 24.9 ± 0.13 | 24.6 ± 0.22 |
| Collagen | - | 18.6 ± 0.22 | 18.0 ± 0.08 |
| Collagen | ADP | 18.5 ± 0.20 | 18.7 ± 0.11 |
| Collagen | thrombin | 18.8 ± 0.17 | 18.2 ± 0.15 |
| Laminin | - | 24.8 ± 0.21 | 15.5 ± 0.18* |
| Laminin | ADP | 25.2 ± 0.11 | 23.8 ± 0.10 |
| Laminin | thrombin | 25.7 ± 0.19 | 24.0± 0.12 |
| CRP | - | 21.9 ± 0.18 | 15.8 ± 0.21* |
| CRP | ADP | 23.8 ± 0.21 | 24.3 ± 0.09 |
| CRP | thrombin | 24.6 ± 0.19 | 25.1 ± 0.18 |
Purified human and wild-type (WT) and Scar/WAVE-1-/-murine platelets (2 × 107/ml) were placed on coverslips coated with fibrinogen, collagen, laminin or collagen-related peptide (CRP) for 45 min at 37°C. In selected experiments, platelets were treated with 1 U/ml thrombin or 10 μM ADP. Values are reported as follows: platelet surface area (SA) = mean ± SEM of at least 200 cells.
P < 0.05 with respect to WT.
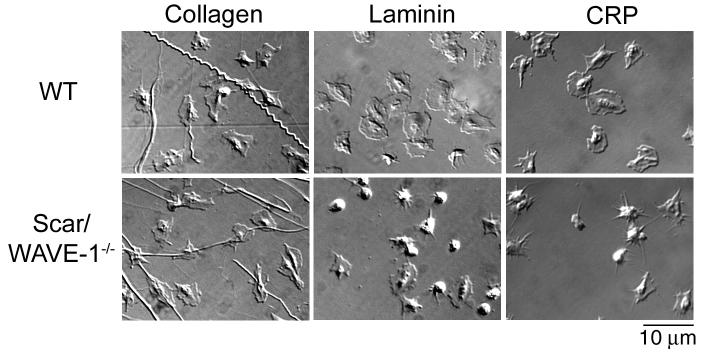
Wild type platelets generate extensive lamellipodia on collagen, laminin and the GPVI-specific peptide, CRP, in the absence of GPCR agonists (Fig. 3). Strikingly, there is a marked reduction in formation of lamellipodia of Scar/WAVE-1-/- platelets on CRP and on laminin (which signals via α6β1 and GPVI [15]) accompanied by formation of prominent filopodia (most notably on CRP), whereas the response on collagen was not changed. These changes resulted in a marked reduction in ‘spreading’ on CRP and laminin but not on collagen as determined by measurement of surface area (Table 2). It is noteworthy, however, that Scar/WAVE-1-/- platelets retain the ability to form filopodia on CRP and laminin (Fig. 3), and that full spreading is restored by addition of either thrombin or ADP (Table 2). The lack of a detectable change in formation of lamellipodia on collagen could be due to the ability of the integrin α2β1 and the feedback agonists, ADP and thromboxane A2, to mediate lamellipodia assembly [19].
Fig. 3.
Role of Scar/WAVE-1 in GPVI-murine platelet spreading. Purified wild-type (WT) and Scar/WAVE-1-/- murine platelets (2 × 107/ml) were placed on coverslips coated with collagen, laminin or collagen-related peptide (CRP) for 45 min and imaged using DIC microscopy. Results are representative of at least 3 experiments.
GPVI-mediated platelet aggregation is impaired in the absence of Scar/WAVE-1
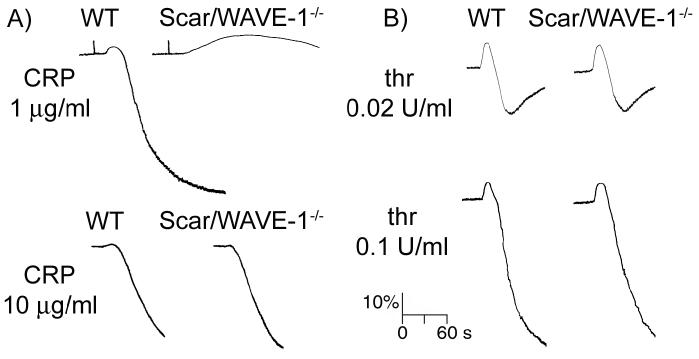
The role of Scar/WAVE-1 in the process of agonist-induced platelet shape change and aggregation was examined by either measuring the optical density of a platelet suspension using a Born aggregometer or by quantifying the number of agonist-induced platelet aggregates using a Coulter counter, a method known as single platelet counting [20]. The initial increase in optical density that is observed in a Born aggregometer correlates to platelet spheration (shape change) while a decrease is indicative of platelet aggregation. As shown in Fig. 4, Scar/WAVE-1-/- platelets showed a dramatically prolonged platelet shape change and a virtual blockage of aggregation in response to low concentrations of the GPVI-specific agonist CRP. A decrease in aggregation was also identified through single platelet counting in response to 1 μg/ml CRP (75.1 ± 5.8 % WT platelets vs 42.3 ± 5.2 % Scar/WAVE-1-/- platelets in aggregates). It is noteworthy that the response to higher concentrations of CRP (10 μg/ml) was not altered in the absence of Scar/WAVE-1 (Fig. 4). A similar shift in the dose-dependent response curve was observed for collagen, whereby platelet aggregation to a low concentration of collagen was slightly reduced in the absence of Scar/WAVE-1 as measured by single platelet counting (1 μg/ml collagen: 68.4 ± 4.2 % WT platelets vs 56.2 ± 15.6 % Scar/WAVE-1-/- platelets in aggregates), whereas the response to a higher concentration was not altered (10 μg/ml collagen: 91.9 ± 3.0 % WT platelets vs 92.5 ± 4.2 % Scar/WAVE-1-/- platelets in aggregates), a result that was confirmed by measurement of Born aggregometry (data not shown). In contrast, the response to threshold (0.02 U/ml) or intermediate (0.1 U/ml) concentrations of thrombin was similar for wild-type and Scar/WAVE-1-/- mouse platelets (Fig. 4).
Fig. 4.
Role of Scar/WAVE-1 in platelet aggregation to GPVI and G-protein coupled agonists. Washed platelets (2 × 108/ml) from wild-type (WT) and Scar/WAVE-1-/- mice were stimulated with A) CRP or B) thrombin (thr) at the indicated concentrations, and the change in optical density indicative of aggregation recorded. One representative experiment of three separate trials is depicted.
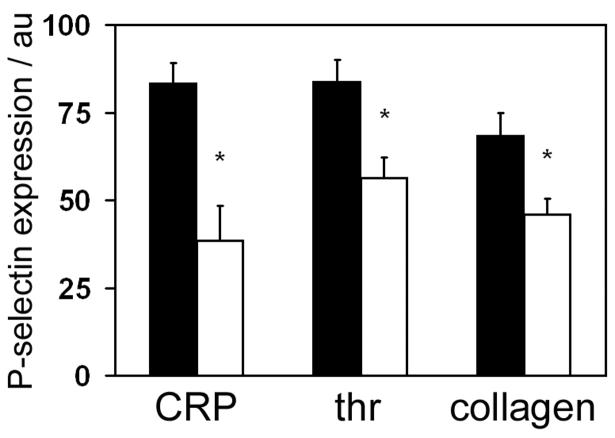
The role of Scar/WAVE-1 in platelet secretion downstream of GPVI and G-protein coupled receptors was investigated by determining P-selectin exposure to CRP, thrombin and collagen. In the absence of Scar/WAVE-1, P-selectin exposure induced by maximally-effective concentrations of CRP, thrombin and collagen was reduced by between 30 and 50% relative to that in wild-type platelets (Fig. 5A), with a similar level of inhibition observed at lower concentrations of all three agonists (data not shown). The observation that the level of secretion is inhibited by maximally-effective concentrations of the three agonists indicates a possible reduction in the level of P-selectin in mutant platelets or a possible secretion defect.
Fig. 5.
Role of Scar/WAVE-1 in platelet P-selectin exposure.
Washed platelets (2 × 107/ml) from wild-type (black bars) and Scar/WAVE-1-/- (white bars) stimulated with 10 μg/ml CRP, 0.1 U/ml thrombin (thr) or 30 μg/ml collagen for 15 min at 37°C. The degree of FITC-conjugated anti-P-selectin mAb was recorded via FACS analysis in arbitrary units (au). Values are mean ± SEM from 3 experiments. * P < 0.01 with respect to wild-type.
The role of Scar/WAVE-1 in aggregate formation under shear
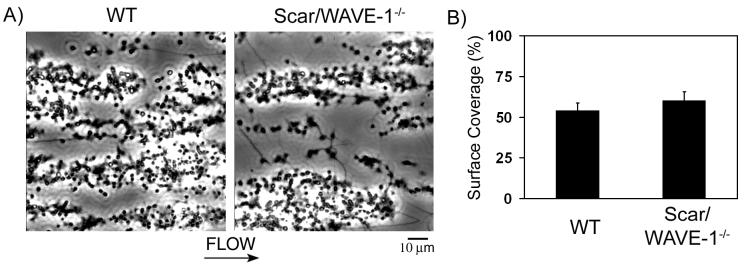
The functional role of Scar/WAVE-1 on platelet adhesion and platelet aggregation on collagen under shear conditions was investigated using an in vitro flow-based assay. Whole blood from wild-type and Scar/WAVE-1-/- mice was perfused over a collagen-coated surface at the arterial shear rate of 1000 s-1 for 4 min. Blood from Scar/WAVE-1-/- mice exhibited robust formation of densely packed platelets on collagen, which was not significantly different from control platelets (Fig. 6). This was confirmed by analysis of surface area coverage by platelets (53.7 ± 5.1 % vs. 60.0 ± 5.7 % surface coverage for control and Scar/WAVE-1-/-, respectively; n=3) and quantification of protein levels of lysates from the microcapillary tubes (data not shown). These results indicate that Scar/WAVE-1 is not required for platelet adhesion and aggregate formation on collagen under flow.
Fig. 6.
Role of Scar/WAVE-1 in platelet adhesion and aggregate stability on collagen under flow. Mouse blood anticoagulated with PPACK and heparin was perfused through a collagen-coated microslide at a shear rate of 1000 s-1 for 4 min followed by modified Tyrode buffer for 3 min to remove non-adherent cells. Subsequently, slides were visualized using phase-contrast microscopy. A) Representative images of platelet adhesion from wild-type (left panel) and Scar/WAVE-1-/- (right panel) mice are shown. Direction of flow is from left to right. Images are representative of 3 experiments. B) Platelet adhesion results are expressed as mean ± SEM of surface area covered by platelets.
DISCUSSION
The Wiskott-Aldrich syndrome protein (WASP) family of proteins have been implicated as major signalling proteins to effect the nucleation of actin assembly by Arp2/3 complex via activation of small GTPases [21]. There are five WASP-family proteins in the human and mouse genomes: WASP, N-WASP and Scar/WAVE-1-3. WASP and N-WASP have previously been shown to have a negligible role in actin assembly and Arp2/3 complex activation in platelets [22, 23]. Human platelets express Scar/WAVE-1 and Scar/WAVE-2, and possibly a low level of Scar/WAVE-3 [11, 12]. Mouse platelets express Scar/WAVE-1 and Scar/WAVE-2, although it is not known if they also express Scar/WAVE-3. Although Scar/WAVE isoforms have unique tissue distributions [24], they are frequently co-expressed and exhibit redundancy of function [12-14].
The inability of Scar/WAVE-1-/- platelets to form lamellipodia on the GPVI-specific ligand CRP and on laminin (which also signals via GPVI [15]), and the severely impaired aggregation and shape change response to CRP and collagen demonstrates a critical role for Scar/WAVE-1 in GPVI receptor signalling. However, this would appear to be at variance with the observation that Scar/WAVE-1-/- platelets retain the ability to spread on collagen. This may be explained by the ability of collagen to signal via integrin α2β1, or by a net increase in affinity of collagen for GPVI as a consequence of binding to the integrin, thereby enabling collagen to serve as a more powerful platelet agonist [25]. A similar mechanism may not apply to laminin, which also mediates adhesion and spreading via a β1 integrin (α6β1) and GPVI respectively, because laminin has an approximate ten fold lower affinity for GPVI [15] or alternatively α6β1 may generate a weaker signal than α2β1. Alternatively, Scar/WAVE-1 may play an important role within the secretion process downstream of GPVI, or perhaps Scar/WAVE-1-/- mice have a developmental defect leading to a possible reduction in granule formation and/or vesicle transport.
These results suggest that the tyrosine kinase-linked receptor GPVI may mediate formation of lamellipodia via Scar/WAVE-1 and that GPCR agonists signal via Scar/WAVE-2. Indeed, this may also hold true for fibrinogen and thereby explain the increase in filopodia formation observed in a subpopulation of Scar/WAVE-1-/- on fibrinogen. If this is the case, it would suggest that Scar/WAVE-1 is selectively regulated downstream of tyrosine phosphorylation-based signalling pathways. Tyrosine phosphorylation of Scar/WAVE proteins has been previously detected in other cell types [26], but platelets appear to lack the Abl tyrosine kinases and we have not been able to detect tyrosine phosphorylation of Scar/WAVE-1 in CRP-stimulated human and mouse platelets (data not shown), suggesting that there may be major differences between platelets and other cell types in tyrosine kinase signalling to Scar/WAVE.
The absence of Scar/WAVE-1 had no major defect in lamellipodia formation on fibrinogen in the presence of GPCR agonists. Similar to the Rac1-/- platelets [3], a proportion of Scar/WAVE-1-/- platelets have abnormally long filopodia on fibrinogen, suggesting altered signalling via Rac to normal actin structures. This could be a shift in the balance between branched lamellipodial structures and long unbranched filopodia, as reported for gelsolin-/- platelets [2]. Gelsolin is thought to promote the generation of new filament barbed ends, which are conducive to Arp2/3 complex induced actin assembly in lamellipodia [27].
We have previously reported that Rac1 plays a critical role in lamellipodia formation in platelets in response to both matrix proteins and GPCR agonists [3]. Further, the absence of Rac1 causes a reduction in thrombus size on collagen at arterial rates of flow due to an increase in embolisation [3]. In the present study, thrombus formation of Scar/WAVE-1-/- platelets on collagen is not altered, which is consistent with the observation that lamellipodia formation on collagen under static conditions is not altered. Further, this is in agreement with a previous report that a reduction in the level of GPVI to 20% of its normal level has no effect on the ability of the platelets to from stable thrombi on collagen even though thrombus formation is abolished in the absence of GPVI [28]. This demonstrates that a marked reduction in the strength of signalling by GPVI does not cause a signification reduction in thrombus formation or stability under flow conditions, presumably because of the compensatory roles of ADP and thromboxanes, which presumably induce lamellipodia formation through Scar/WAVE-2 [10,29]. The creation of a double knockout of Scar/WAVE-1 and Scar/WAVE-2 would address this, although this would have to be conditional as the Scar/WAVE-2 knockout is embryonically lethal [10,18,29,30]. In mouse embryo fibroblasts, Scar/WAVE1 knockout has little or no effect on lamellipodia formation or spreading on fibronectin [14], while Scar/WAVE2 knockout appears to significantly affect lamellipodia formation [10,29].
An important consideration is whether the abolition of lamellipodia formation and aggregation to CRP is because of the critical role of Scar/WAVE-1 in regulating the actin cytoskeleton or the result of an additional role of Scar/WAVE-1 in the GPVI signalling cascade. The low yield of platelets from the Scar/WAVE-1-/- mice at 3 – 4 weeks prevented a detailed analysis of the GPVI signalling events. However, it seems reasonable to assume that the defect in aggregation to CRP in the Scar/WAVE-1-/- mice is mediated through the same pathway as that which results in an abolition of response to low concentrations of CRP in Rac1-/- mice, with partial recovery observed at higher concentrations. Studies in Rac1-/- however demonstrated that the inhibition of aggregation was not mediated by a decrease in tyrosine phosphorylation of Syk and PLCγ2, or decrease in mobilisation of intracellular Ca2+ in the presence of 10 μg/ml CRP [3]. Further studies are required to establish the molecular basis of the decrease in aggregation to CRP in the absence of Rac1 or Scar/WAVE-1.
The absence of Scar/WAVE-1 did not result in an alteration in the number of red blood cells and platelets, or mean platelet volume. This is in agreement with data from the Rac1-/- mice, the upstream activator of Scar/WAVE-1 [3]. Rac deficient fibroblasts show nearly normal migration and chemotaxis [31], suggesting that the Rac- Scar/WAVE pathway may not be essential for all forms of cell migration in development, such as the migration of progenitor cells from the osteoblastic to the vascular niche[32]. It is also a noticeable difference to the WASP-/- mice in which platelet production is impaired [33]. It is noteworthy that leukocyte production was markedly reduced within Scar/WAVE-1-/- mice, indicating a possible role for Scar/WAVE-1 in leukocyte production.
In conclusion we have shown that Scar/WAVE-1 plays an important role downstream of GPVI but not by GPCR agonists in mediating platelet aggregation and lamellipodia formation. This raises the possibility that Scar/WAVE-1 plays a selective role in mediating platelet activation downstream of tyrosine kinase-linked receptor signalling pathways.
ACKNOWLEDGMENTS
L.M. Machesky is supported by a Senior Research Fellowship from the Medical Research Council G117/569 and a British Heart Foundation project grant PG/04/108. O.J.T. McCarty is supported by an American Heart Association Beginning Grant-in-Aid. S.P. Watson is a British Heart Foundation Professor. We thank Seung Kwak at Wyeth-Ayerst (Princeton, NJ) for the gift of Scar/WAVE-1-/- mice. We thank H. Morris for the genotyping of the mice.
REFERENCES
- 1.Barkalow KL, Italiano JE, Jr., Chou DE, Matsuoka Y, Bennett V, Hartwig JH. Alpha-adducin dissociates from F-actin and spectrin during platelet activation. J Cell Biol. 2003;161:557–70. doi: 10.1083/jcb.200211122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falet H, Barkalow KL, Pivniouk VI, Barnes MJ, Geha RS, Hartwig JH. Roles of SLP-76, phosphoinositide 3-kinase, and gelsolin in the platelet shape changes initiated by the collagen receptor GPVI/FcR gamma-chain complex. Blood. 2000;96:3786–92. [PubMed] [Google Scholar]
- 3.McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, Ruf S, Henderson RB, Tybulewicz VL, Machesky LM, Watson SP. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem. 2005;280:39474–84. doi: 10.1074/jbc.M504672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barkalow K, Witke W, Kwiatkowski DJ, Hartwig JH. Coordinated regulation of platelet actin filament barbed ends by gelsolin and capping protein. J Cell Biol. 1996;134:389–99. doi: 10.1083/jcb.134.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992;118:1421–42. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartwig JH, Bokoch GM, Carpenter CL, Janmey PA, Taylor LA, Toker A, Stossel TP. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–53. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 7.Machesky LM, Insall RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8:1347–56. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 8.Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, Hall ME, Pollard TD. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci U S A. 1999;96:3739–44. doi: 10.1073/pnas.96.7.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–41. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan C, Martinez-Quiles N, Eden S, Shibata T, Takeshima F, Shinkura R, Fujiwara Y, Bronson R, Snapper SB, Kirschner MW, Geha R, Rosen FS, Alt FW. WAVE2 deficiency reveals distinct roles in embryogenesis and Rac-mediated actin based motility. EMBO J. 2003;22:3602–12. doi: 10.1093/emboj/cdg350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashiwagi H, Shiraga M, Kato H, Honda S, Sako M, Kurata Y, Kanakura Y, Tomiyama Y. Expression and subcellular localization of WAVE isoforms in the megakaryocyte/platelet lineage. J Thromb Haemost. 2005;3:361–8. doi: 10.1111/j.1538-7836.2004.01082.x. [DOI] [PubMed] [Google Scholar]
- 12.Oda A, Miki H, Wada I, Yamaguchi H, Yamazaki D, Suetsugu S, Nakajima M, Nakayama A, Okawa K, Miyazaki H, Matsuno K, Ochs HD, Machesky LM, Fujita H, Takenawa T. WAVE/Scars in platelets. Blood. 2005;105:3141–8. doi: 10.1182/blood-2003-04-1319. [DOI] [PubMed] [Google Scholar]
- 13.Stovold CF, Millard TH, Machesky LM. Inclusion of Scar/WAVE3 in a similar complex to Scar/WAVE1 and 2. BMC Cell Biol. 2005;6:11. doi: 10.1186/1471-2121-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Legg JA, Bompard G, Dawson J, Morris H, Andrea N, Cooper L, Johnstone SA, Tramountanis G, Machesky LM. N-WASP involvement in dorsal ruffle formation in mouse embryonic fibroblasts. Mol Biol Cell. 2007;18:678–87. doi: 10.1091/mbc.E06-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue O, Suzuki-Inoue K, McCarty OJ, Moroi M, Ruggeri ZM, Kunicki TJ, Ozaki Y, Watson SP. Laminin stimulates spreading of platelets through integrin {alpha}6{beta}1-dependent activation of GPVI. Blood. 2006;107:1406–12. doi: 10.1182/blood-2005-06-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Launay S, Brown G, Machesky LM. Expression of WASP and Scar1/WAVE1 actin-associated proteins is differentially modulated during differentiation of HL-60 cells. Cell Motil Cytoskeleton. 2003;54:274–85. doi: 10.1002/cm.10101. [DOI] [PubMed] [Google Scholar]
- 17.McCarty OJ, Zhao Y, Andrew N, Machesky LM, Staunton D, Frampton J, Watson SP. Evaluation of the role of platelet integrins in fibronectin-dependent spreading and adhesion. J Thromb Haemost. 2004;2:1823–33. doi: 10.1111/j.1538-7836.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- 18.Dahl JP, Wang-Dunlop J, Gonzales C, Goad ME, Mark RJ, Kwak SP. Characterization of the WAVE1 knock-out mouse: implications for CNS development. J Neurosci. 2003;23:3343–52. doi: 10.1523/JNEUROSCI.23-08-03343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue O, Suzuki-Inoue K, Dean WL, Frampton J, Watson SP. Integrin alpha2beta1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCgamma2. J Cell Biol. 2003;160:769–80. doi: 10.1083/jcb.200208043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole A, Gibbins JM, Turner M, van Vugt MJ, van de Winkel JG, Saito T, Tybulewicz VL, Watson SP. The Fc receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. Embo J. 1997;16:2333–41. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machesky LM, Insall RH. Signaling to actin dynamics. J Cell Biol. 1999;146:267–72. doi: 10.1083/jcb.146.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falet H, Hoffmeister KM, Neujahr R, Hartwig JH. Normal Arp2/3 complex activation in platelets lacking WASp. Blood. 2002;100:2113–22. [PubMed] [Google Scholar]
- 23.Gross BS, Wilde JI, Quek L, Chapel H, Nelson DL, Watson SP. Regulation and function of WASp in platelets by the collagen receptor, glycoprotein VI. Blood. 1999;94:4166–76. [PubMed] [Google Scholar]
- 24.Suetsugu S, Yamazaki D, Kurisu S, Takenawa T. Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev Cell. 2003;5:595–609. doi: 10.1016/s1534-5807(03)00297-1. [DOI] [PubMed] [Google Scholar]
- 25.Watson SP, Auger JM, McCarty OJM, Pearce AC. GPVI and integrin alphaIIbbeta3 signalling in platelets. J Thromb Haemost. 2005;3:1752–62. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 26.Leng Y, Zhang J, Badour K, Arpaia E, Freeman S, Cheung P, Siu M, Siminovitch K. Abelson-interactor-1 promotes WAVE2 membrane translocation and Abelson-mediated tyrosine phosphorylation required for Arp2/3 complex function in platelets and fibroblasts. Proc Nat Acad Sci USA. 2005;102:1098–103. doi: 10.1073/pnas.0409120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falet H, Hoffmeister KM, Neujahr R, Italiano JE, Jr., Stossel TP, Southwick FS, Hartwig JH. Importance of free actin filament barbed ends for Arp2/3 complex function in platelets and fibroblasts. Proc Natl Acad Sci U S A. 2002;99:16782–7. doi: 10.1073/pnas.222652499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Best D, Senis YA, Jarvis GE, Eagleton HJ, Roberts DJ, Saito T, Jung SM, Moroi M, Harrison P, Green FR, Watson SP. GPVI levels in platelets: relationship to platelet function at high shear. Blood. 2003;102:2811–8. doi: 10.1182/blood-2003-01-0231. [DOI] [PubMed] [Google Scholar]
- 29.Yamazaki D, Suetsugu S, Miki H, Kataoka Y, Nishikawa S, Fujiwara T, Yoshida N, Takenawa T. WAVE2 is required for directed cell migration and cardiovascular development. Nature. 2003;424:452–6. doi: 10.1038/nature01770. [DOI] [PubMed] [Google Scholar]
- 30.Soderling SH, Langeberg LK, Soderling JA, Davee SM, Simerly R, Raber J, Scott JD. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc Natl Acad Sci U S A. 2003;100:1723–8. doi: 10.1073/pnas.0438033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidali L, Chen F, Cicchetti G, Ohta Y, Kwiatkowski DJ. Rac1-null mouse embryonic fibroblasts are motile and respond to platelet-derived growth factor. Mol Biol Cell. 2006;17:2377–90. doi: 10.1091/mbc.E05-10-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, Hackett NR, Crystal RG, Witte L, Hicklin DJ, Bohlen P, Eaton D, Lyden D, de Sauvage F, Rafii S. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 33.Sabri S, Foudi A, Boukour S, Franc B, Charrier S, Jandrot-Perrus M, Farndale RW, Jalil A, Blundell MP, Cramer EM, Louache F, Debili N, Thrasher AJ, Vainchenker W. Deficiency in the Wiskott-Aldrich protein induces premature proplatelet formation and platelet production in the bone marrow compartment. Blood. 2006;108:134–40. doi: 10.1182/blood-2005-03-1219. [DOI] [PubMed] [Google Scholar]