Abstract
We assessed the neurochemical basis of olfactory learning induced by presentations of odor and moderate shock in infant rats. Paradoxically, shock conditioning produces an odor preference in 8-day-olds, but an odor aversion in 12-day-olds. Studies have demonstrated the importance of opioids in early olfactory learning; their specific role remains undefined. In this study, postnatal Days 8 and 12 pups were systemically injected with naltrexone, a nonspecific opioid antagonist, or saline and received either paired or backward presentations of odor-moderate shock or odor-only presentations. Blocking the opioid system during conditioning disrupted acquisition of the Day 8 odor preference, but not the Day 12 odor aversion. Additional Day 8 pups were given naltrexone posttraining. Naltrexone not only blocked consolidation of an odor preference but also yielded an odor aversion. These results suggest that the opioid system has a critical role in both olfactory learning and consolidation of odor preferences during the sensitive period.
Keywords: opioids, shock conditioning, olfactory learning, consolidation, infant rats, naltrexone, sensitive period
Altricial rodents enter the world without functional visual and auditory sensory systems. The neonate relies on a still-developing olfactory system to respond to the olfactory cues that are critical for survival—the maternal odor and those odors associated with the nest environment. A variety of stimuli that approximate the natural nest conditions, including noxious stimuli, support odor preference formation in neonates (Camp & Rudy, 1988; Johanson & Hall, 1982; Johanson & Teicher, 1980; Pedersen, Williams, & Blass, 1982; Sullivan, Landers, Yeaman, & Wilson, 2000). Some of these stimuli, particularly noxious stimuli, lose their ability to produce odor preferences in older infants, suggesting a sensitive period for odor conditioning during the first 9 days of life (Woo & Leon, 1987; Sullivan et al., 2000; Sullivan & Wilson, 1990). This period also corresponds to when pups begin walking (Bolles & Woods, 1964). The neurochemical basis of this paradoxical infant learning, in which a preference is learned for a noxious stimulus during the sensitive period, is not understood. Many neurotransmitters have important roles in early olfactory learning, and include norepinephrine (Sullivan, Wilson, Lemon, & Gerhardt, 1994; Sullivan, Zyzak, Skierkowski, & Wilson, 1992), serotonin (McLean, Darby-King, Sullivan, & King, 1993; Price, Darby-King, Harley, & McLean, 1998), dopamine (Weldon, Travis, & Kennedy, 1991), glutamate (Weldon & Fedorcik, 1993), GABA (Okutani, Yagi, & Kaba, 1999), and opioids (e.g., Aroyewun & Barr, 1992;Barr & Rossi,1992; Blass & Fitzgerald, 1988; Kehoe, 1988; Petrov, Varlinskaya, Becker, & Smotherman, 1998; Shide & Blass, 1991; Smotherman & Robinson, 1992). Since endogenous opioids mediate physiological and behavioral responses to pain and both rewarding and stressful situations, this neurotransmitter/neuromodulator system was chosen as a candidate system involved in this paradoxical infant learning.
Opioid receptors (μ, κ, and δ) are distributed throughout the brain in both infants and adults. Opiate receptors are present in 14-day-old fetuses and begin reaching adult levels by the 3rd postnatal week (Clendeninn, Petraitis, & Simon, 1976). μ and κ receptors are present at birth while δ receptors are not present until the 2nd postnatal week (Kornblum, Hurlbut, & Leslie, 1987; Petrillo, Tavani, Verotta, Robson, & Kosterlitz, 1987; Spain, Roth, & Coscia, 1985). μ receptors are morphine and enkephalin selective, mediating nociception and reward. δ receptors are enkephalin selective, mediating affective behaviors and are found primarily in the limbic system while κ receptors are dynorphine selective and mediate less-addicting analgesia as well as affective behaviors (reviewed in Kehoe, 1988; Tseng, 1995).
Pairings of morphine with saccharine or odor show that the endogenous opioid system is present and functional in pups as early as 5 days old, and these pairings produce a conditioned preference to the conditioned stimulus (Kehoe & Blass, 1986). In postnatal Day 5 pups, pairings of low doses of morphine with odor produce odor preferences while high doses produce odor aversions (Randall, Kraemer, Dose, Carbary, & Bardo, 1992). Injections of morphine into the ventral tegmental area (an area associated with the adult reward pathway) paired with an odor in pups as young as postnatal Day 4 are sufficient for an odor preference (Barr & Rossi, 1992). Intraoral infusions of sucrose paired with odor produce an odor preference in 6-day-old pups, and both the acquisition and expression of the odor preference is naltrexone reversible, suggesting the role of endogenous opioids in odor preference formation (Shide & Blass, 1991). Similarly, intraoral infusion of milk activates endogenous opioids (Blass & Fitzgerald, 1988; Kehoe, 1988). Other work shows that μ receptors are involved in suckling responses (Petrov et al., 1998) and that nipple-milk conditioning involves endogenous opioids (Robinson, Arnold, Spear, & Smotherman, 1993; Robinson & Smotherman, 1997; Smotherman & Robinson, 1992). Overall, these studies demonstrate a role of endogenous opioids in normal mother–infant attachment.
Our study investigated the role of endogenous opioids using another mammalian model of mother– infant attachment, odor–shock conditioning. This model approximates the unpleasant events that neonates encounter in their natural nest environment, which include rough transport and being stepped upon when the mother enters and leaves the nest. Specifically, postnatal Days 8 and 12 pups were given systemic injections of naltrexone, a nonspecific opioid antagonist, or equal volumes of saline before odor–shock conditioning. Additionally, in a separate experiment, Day 8 pups were first conditioned and then given systemic injections of naltrexone or saline. All subjects were later tested in a behavioral Y-maze for a subsequent odor preference or aversion.
EXPERIMENT 1: PARADOXICAL ODOR–SHOCK CONDITIONING
Odor–shock conditioning in postnatal Day 9 pups and younger produces a paradoxical odor preference (Camp & Rudy, 1988; Sullivan et al., 2000). Pups older than 9 days show a subsequent odor aversion after the same training paradigm. There are no differences between shock thresholds in these ages (Emerich, Scalzo, Enters, Spear, & Spear, 1985; Haroutunian & Campbell, 1979; Sullivan et al., 2000). Our purpose in Experiment 1 was to replicate previous results and to extend preference-conditioning results to a new behavioral test, which requires the subject to climb either upon or over a barrier to demonstrate an odor preference.
Methods
Subjects
Male and female pups, born of Long-Evans rats (Harlan Sprague-Dawley, Indianapolis, IN) in the animal vivarium at the University of Oklahoma, were used. Dams were housed in polypropylene cages with wood shavings, and kept in an environment with controlled temperature (23°C) and light (12:12 hr light:dark). Food and water were available ad libitum. All procedures were approved by the University of Oklahoma Institutional Animal Care and Use Committee, which follows standards certified by the National Institutes of Health Guide for Care and Use of Laboratory Animals 1985. Subject numbers used in each experimental condition are listed in the figure captions of the corresponding figures.
Training for Experiment 1A
A total of 33 pups derived from seven litters were used in Experiment 1A. Postnatal Days 8 (18.1–20.9 g) and 12 (20.3–30.9 g) pups were removed from each litter (Only healthy pups with similar weights were chosen from each litter.) and randomly assigned to a training condition: (a) paired odor–shock, (b) backward odor–shock, and (c) odor only. Pups were marked for identification using indelible ink. During a 1 hr training session, pups received 14 presentations of a 30-s peppermint odor (conditioned stimulus) and a 1-s 0.5 mA tail shock (unconditioned stimulus), with an intertrial interval of 4 min. Paired odor–shock subjects received 14 pairings of the 30-s odor with shock during the last 29 s while backward odor–shock subjects received a 1-s shock 2 min after an odor presentation. Odor-only subjects received a 30-s presentation of the peppermint odor. Peppermint odor was presented with a flow-dilution olfactometer at 2 L/min and at a concentration of 1:10 peppermint vapor. Pups were trained in 600-ml glass beakers and given a 10-min habituation period in these beakers prior to beginning training to recover from experimenter handling. Following training, pups were returned to their mother until tested.
Behavioral Testing in a Y-Maze
Pups were tested with a behavioral Y-maze the following day. The Y-maze consisted of a habituation chamber (7 cm long and 9 cm wide) with two alleys (22 cm long and 9 cm wide) extending at 45-degree angles. One arm of the maze contained pine wood odor (20 ml in petri dish) while the other arm contained the peppermint odor (25 μl of peppermint extract placed on a kimwipe for 5 min in a ventilation hood). Each pup was placed in the starting chamber and given 5 s for habituation before the doors to the alleys were removed. Each subject had 60 s to make a choice, and a choice was counted when a pup had placed its nose 3 cm or 6 cm down the alley, for Days 8 and 12 pups, respectively. Each subject was given five trials, and the floor was wiped clean (cloth with water) between each trial. A 30-s intertrial was used between testing trials, and the orientation of the pup when placed in the habituation chamber was counterbalanced between trials. Observations of each pup were made without knowledge of the training condition.
Training for Experiment 1B
A total of 23 pups derived from four litters were used in Experiment 1B. Postnatal Day 8 pups were trained with the same protocol followed in Experiment 1A, with the exception that pups were trained in 400-ml beakers, and the odor was presented with a flow-dilution olfactometer at 1 L/min and at a concentration of 1:10 peppermint vapor.
Behavioral Testing in a Two-Odor-Choice Climbing Test
Pups were tested on postnatal Day 10 with a two-odor-choice climbing test. The testing apparatus consisted of a Plexiglas arena (23.5 cm long × 15 cm wide) placed on a metal tray. The arena was divided into three chambers (7 cm, 5.5 cm, and 7 cm) via wooden bars (1 cm high × 2 cm wide) covered in aluminum foil to prevent absorption of any odor. One 7-cm side contained 100 ml of clean wood shavings, and the other 7-cm side contained 100 ml of shavings treated with peppermint odor (0.05 ml of peppermint extract, placed in a ventilation hood for 15 min). Each pup was placed in the 5.5-cm starting chamber (the area between the two odor sides) and given 60 s, in which the total number of choices to either side and the total time in either side (if the pup crawled over the barrier) were recorded. Criteria for a choice consisted of placement of the head and/or front paws onto the wooden bars or crawling over the barriers into the shavings. Each pup received two trials, with the orientation of the pup counterbalanced between trials. The floor was wiped clean between trials, and a 30-s intertrial was used between testing trials. The total number of choices made towards the odor for the two trials was summed and divided by the total number of choices made towards both odors (preference ratio). Observations of each pup were made without knowledge of the training condition.
Analysis
We used the analysis of variance test (ANOVA) and post hoc Fischer tests to analyze differences between training conditions.
Results
Y-Maze: Postnatal Day 8 Subjects Receiving Paired Presentations of Odor–Shock Showed Significantly Greater Number of Choices Towards the Conditioned Odor
ANOVA analysis revealed a main effect of training condition, F(2, 13) = 4.69, p<0.03 (Fig. 1A). Post hoc tests showed that subjects receiving paired odor–shock conditioning had significantly more choices towards the conditioned odor during the Y-maze test compared to subjects that received backward odor–shock or odor-only conditioning, p<0.02. Post hoc tests showed no difference between the number of choices from backward odor– shock and odor-only subjects.
FIGURE 1.
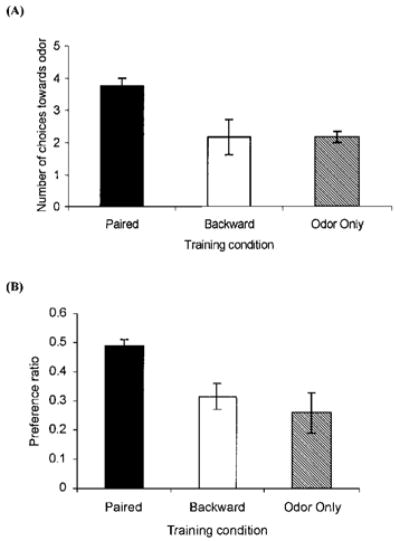
Number of approaches towards the conditioned odor for postnatal Day 8 rat pups receiving either paired (n = 4) or backward (n = 6) odor–shock presentations, or odor-only (n = 6) presentations in Experiment 1A. Bars represent mean values; vertical lines indicate SEM. Paired presentations of odor-moderate shock resulted in an increased number of choices towards the peppermint odor (A). Preference ratio for postnatal Day 8 rat pups receiving either paired (n = 7) or backward (n = 8) odor–shock, or odor-only (n = 8) presentations in Experiment 1B. Bars represent mean values, vertical lines indicate SEM. Paired presentations resulted in an increased number of choices made towards the peppermint odor (B).
Two-Odor-Choice Climbing Test: Postnatal Day 8 Subjects Receiving Paired Presentations of Odor– Shock Showed a Significant Preference Ratio Towards the Conditioned Odor
ANOVA analysis revealed a main effect of training condition, F(2, 20) = 5.25, p<0.02 (Figure 1B). Post hoc tests showed that subjects receiving paired odor–shock training had significantly more choices towards the conditioned odor compared to the subjects receiving backward odor–shock or odor-only conditioning, p<0.03. In addition, 4 of the 7 paired subjects climbed over the wooden barrier and spent time exploring the peppermint treated shavings (data not shown). Post hoc tests showed no difference between the number of choices from backward odor–shock or odor-only subjects, and none of the control pups climbed over the barrier into the peppermint-scented shavings.
Y-Maze: Postnatal Day 12 Subjects Receiving Paired Presentations of Odor–Shock Made Significantly Fewer Choices Towards the Conditioned Odor
Similarly, ANOVA analysis revealed a main effect of training condition with Day 12 subjects, F(2, 14) = 6.90, p<0.01 (Fig. 2). Post hoc tests revealed that paired odor–shock subjects chose the conditioned odor significantly less than backward odor–shock or odor-only subjects, p<0.02. No difference in the number of choices towards the odor was found between backward odor–shock and odor-only subjects.
FIGURE 2.

Number of approaches towards the conditioned odor for postnatal Day 12 rat pups receiving either paired (n = 6) or backward (n = 5) odor–shock presentations, or odor-only (n = 6) presentations in Experiment 1A. Bars represent mean values; vertical lines indicate SEM. Paired presentations of odor-moderate shock significantly decreased the number of choices made towards the peppermint odor.
EXPERIMENT 2: THE ROLE OF OPIOIDS DURING LEARNING
Experiment 1 replicated results that postnatal Day 8 pups receiving paired presentations of odor and moderate shock learn a subsequent odor preference while postnatal Day 12 pups receiving the same treatment learn a subsequent odor aversion. Control subjects in both age groups show neither a preference nor aversion towards the odor. In addition, results indicated the newly designed behavior test may provide a more stringent testing tool for future experiments, as paired-treated subjects demonstrated a significant number of approaches (a preference) towards the odor, with some subjects even climbing over the barrier. Similar to Y-maze results, control subjects in the new test showed neither a preference nor aversion towards the odor. The purpose of Experiment 2 was to examine the role of endogenous opioids during odor preference and aversion acquiccsition (learning) by use of the nonspecific opioid antagonist, naltrexone.
Methods
Training and Testing
A total of 73 pups derived from 15 litters were used in Experiment 2. Postnatal Days 8 (15.9–20.2 g) and 12 (25.1–31.4 g) subjects were given systemic injections of naltrexone (Naltrexone HCl, Sigma Chemical, St. Louis, MO) or equal volume saline before training in the conditioning paradigm described in Experiment 1A. Subjects received 0.5 mg/kg of naltrexone (Kehoe & Blass, 1986) and were given 15 min to recover undisturbed in an incubator (27°C) before initiating the training protocol. We used a 0 (no movement) to 5 (movement of all five extremities) rating scale to analyze conditioned behavioral activation 10 s before and during presentation of the odor (Hall, 1979). After training, pups were returned to the mother and tested in a Y-maze the following day. Observations of each pup were made without knowledge of the training condition.
Analysis
We used ANOVA and post hoc Fischer tests to analyze both acquisition and testing data. Behavior recorded both before and during the odor presentation was summed in blocks of two trials before analysis.
Results
Postnatal Day 8 Naltrexone Blocked Learning of an Odor Preference
ANOVA analysis revealed a significant effect of treatment, F(5, 27) = 5.58, p<0.01 (Fig. 3). Post hoc Fischer tests revealed that naltrexone significantly reduced the number of choices towards the conditioned odor in the paired training condition, p<0.01. Paired naltrexone subjects approached the conditioned odor in the same manner as did the backward and odor-only subjects. Naltrexone had no significant effect in the backward odor–shock or odor-only training conditions. No other differences were found. Analysis of learning acquisition behavior indicated that all subjects had similar preconditioning behavior, F(5, 30) = 1.25, p > 0.18 (Figure 4A). However, analysis of conditioned behavior indicated a significant effect of treatment, F(5, 30) = 2.37, p<0.01 (Fig. 4B). Post hoc tests showed that paired saline subjects had significant conditioned behavioral activation in response to the odor compared to paired naltrexone subjects as well as all other subjects, p<0.01. Paired naltrexone subjects were only different from the two odor-only conditions, p<0.02. Naltrexone had no effect in backward odor–shock and odor-only conditions.
FIGURE 3.
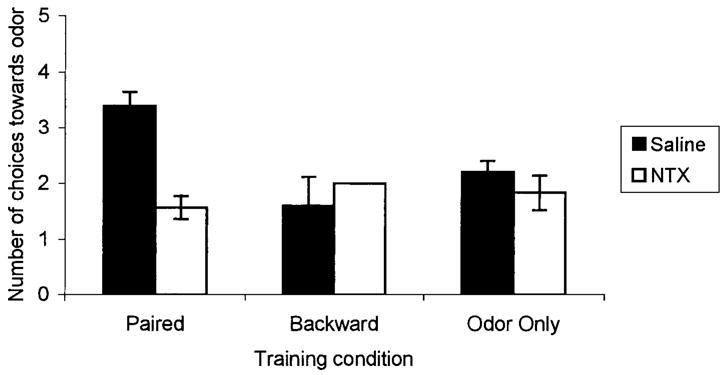
Number of choices towards the conditioned odor for postnatal Day 8 rat pups receiving either paired (Sal: n = 5, NTX: n = 7) or backward (Sal: n = 5, NTX: n = 5) odor–shock presentations, or odor-only (Sal: n = 5, NTX: n = 6) presentations in Experiment 2. Bars represent mean values; vertical lines indicate SEM. The backward NTX condition has no SEM. Blocking opioids during paired presentations of odor-moderate shock resulted in significantly fewer choices made towards the peppermint odor. Sal = saline; NTX = naltrexone.
FIGURE 4.
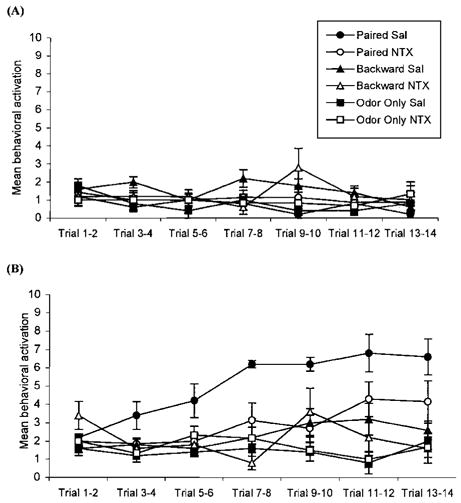
Pre-odor behavioral activation for postnatal Day 8 rat pups receiving either paired or backward odor– shock presentations, or odor-only presentations in Experiment 2. Each data point represents the summation of behavior from two consecutive trials; vertical lines indicate SEM. Conditioning treatment had no effect on behavioral activation before odor presentations (A). Conditioned behavioral activation. The presence of an opioid antagonist during paired presentations of odor and shock disrupted learning (B).
Postnatal Day 12: Naltrexone Did Not Block Learning of an Odor Aversion
Analysis of the results from conditioning after the sensitive period showed a significant effect of treatment, F(5, 34) = 3.67, p<0.01 (Fig. 5). In contrast to the results from postnatal Day 8 training, post hoc tests revealed that paired saline and paired naltrexone subjects had no significant difference in their response to the conditioned odor, p > 0.78. Both paired saline and paired naltrexone subjects approached the odor significantly less than all other subjects, p<0.04. Naltrexone had no effect in the backward odor–shock and odor-only training conditions. Analysis of learning acquisition behavior indicated that all subjects had similar preconditioning behavior, F(5, 30) = 0.89, p > 0.63 (Fig. 6A). Analysis of conditioned behavior revealed a significant effect of treatment, F(5, 30) = 3.10, p<0.01 (Fig. 6B). Post hoc tests revealed that paired saline subjects compared to paired naltrexone subjects had a significant difference in conditioned behavior, p<0.01. However, both paired saline and paired naltrexone subjects were significantly different from all other subjects, p<0.01. Naltrexone had no effect in the backward odor–shock and odor-only conditions.
FIGURE 5.
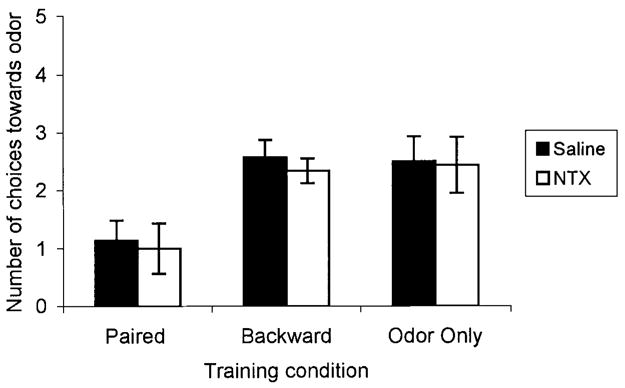
Number of choices towards the conditioned odor for postnatal Day 12 rat pups receiving either paired (Sal: n = 7, NTX: n = 7) or backward (Sal: n = 7, NTX: n = 6) odor–shock presentations, or odor-only (Sal: n = 6, NTX: n = 7) presentations in Experiment 2. Bars represent mean values; vertical lines indicate SEM. Blocking opioids during paired presentations of odor-moderate shock did not effect the number of choices made towards the peppermint odor compared to saline controls. Sal = saline; NTX = naltrexone.
FIGURE 6.
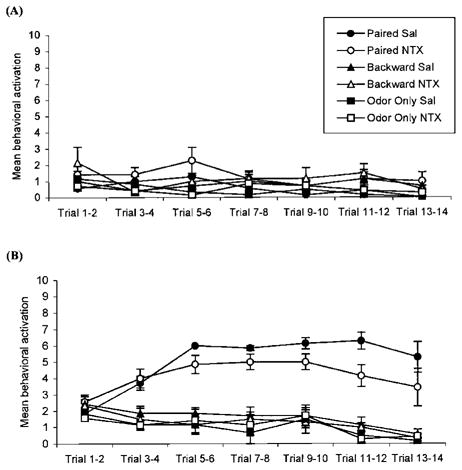
Pre-odor behavioral activation for postnatal Day 12 rat pups receiving either paired or backward odor– shock presentations, or odor-only presentations in Experiment 2. Each data point represents the summation of behavior from two consecutive trials; vertical lines indicate SEM. Conditioning treatment had no effect on behavioral activation before odor presentations (A). Conditioned behavioral activation. Paired presentations of odor and shock in the presence of an opioid antagonist in postnatal Day 12 pups did not disrupt learning (B).
EXPERIMENT 3: THE ROLE OF OPIOIDS DURING CONSOLIDATION
Results from Experiment 2 suggested that blocking endogenous opioids during training affects the animal’s ability to acquire a learned odor preference, but not an odor aversion. The purpose of Experiment 3 was to distinguish the role of opioids in acquisition and consolidation in postnatal Day 8 pups.
Methods
Training and Testing
A total of 44 pups derived from eight litters were used in Experiment 3. Immediately following training (with the same protocol used in the previous experiments), postnatal Day 8 pups (15.6–19.9 g) were given systemic injections of naltrexone (0.5 mg/kg) or equal volume saline, and allowed to recover from injections and handling for 5 min (in an incubator at 27°C) before being returned to the mother. On the following day, pups were tested in a Y-maze, and the observer was blind to the training condition.
Results
Blocking Opioids After Conditioning Blocked Consolidation of an Odor Preference and Permitted an Odor Aversion
ANOVA analysis revealed a significant effect of treatment, F(5, 38) = 12.01, p<0.01 (Fig. 7). Post hoc tests showed that paired saline subjects chose the conditioned odor significantly more times than all other subjects, p<0.01, while paired naltrexone subjects chose the conditioned odor significantly less times than all other subjects, p<0.03. Naltrexone had no effect in backward odor–shock or odor-only conditioning. Analysis of preconditioning behavior showed no differences, F(5, 30) = 0.73, p > 0.84 (Fig. 8A) while conditioned behavior was significantly affected by treatment, F(5, 30) = 13.00, p<0.01 (Figure 8B). Post hoc analysis showed no difference between behavioral conditioning in the paired saline and paired naltrexone groups, p > 0.66, and both paired saline and paired naltrexone subjects had significant conditioned behavioral activation compared to all other subjects, p<0.01.
FIGURE 7.
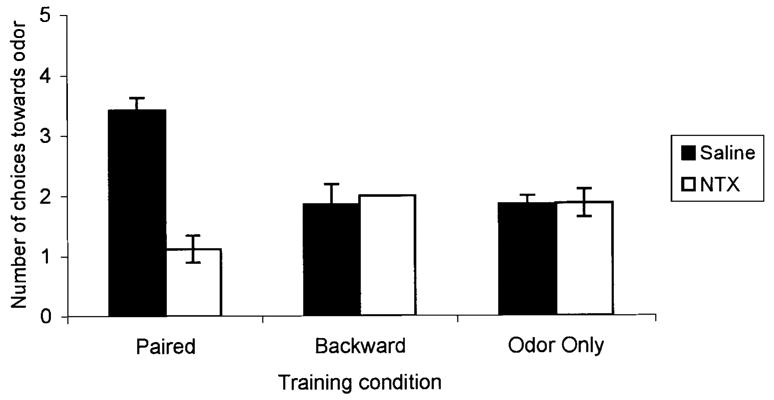
Number of choices towards the conditioned odor for postnatal Day 8 rat pups receiving either paired (Sal: n = 7, NTX: n = 8) or backward (Sal: n = 7, NTX: n = 7) odor–shock presentations, or odor-only (Sal: n = 7, NTX: n = 8) presentations in Experiment 3. Bars represent mean values; vertical lines indicate SEM. The backward NTX condition has no SEM. Blocking opioids after paired presentations of odor and moderate shock significantly reduced the number of choices made towards the peppermint odor. Sal = saline; NTX = naltrexone.
FIGURE 8.
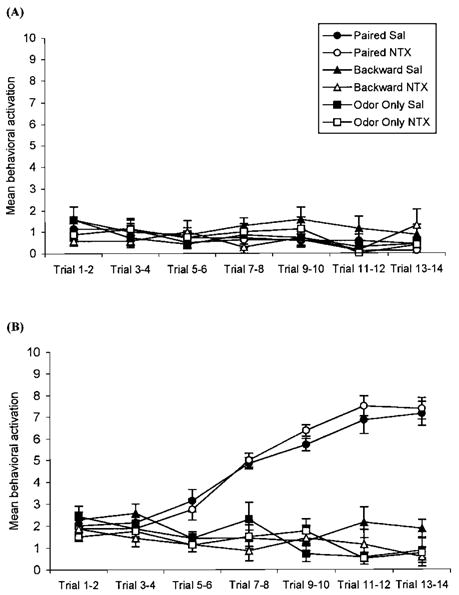
Pre-odor behavioral activation for postnatal Day 8 rat pups receiving either paired or backward odor– shock presentations, or odor-only presentations in Experiment 3. Each data point represents the summation of behavior from two consecutive trials; vertical lines indicate SEM. Conditioning treatment had no effect on behavioral activation before odor presentations (A). Conditioned behavioral activation. Paired presentations of odor and shock in both groups of subjects produced conditioned behavioral activation equally, thus, the effects of naltrexone after conditioning can be interpreted as affecting consolidation (B).
GENERAL DISCUSSION
Overall, these results suggest that the endogenous opioid system is critical for odor preference formation during the sensitive period. Opioids appear to have a dual role in learning during the neonatal sensitive period: (a) Opioids function in developing an odor preference, and (b) opioids function in consolidation of the preference. In sharp contrast, opioids do not appear to have a critical role in acquisition of an odor aversion after the sensitive period. However, it is quite possible that using more stringent behavioral tests to detect differences in aversion levels may produce a different outcome. Additionally, close examination of the acquisition data from Experiment 2 (Fig. 4B) indicates that additional training trials may support acquisition of an odor preference. However, this was not tested, and the effect of blocking opioids during learning is apparent from the differences in the acquisition curves between naltrexone and saline-treated subjects.
Other labs have investigated the role of specific opioid receptors in conditioning using other models, such as nipple-milk conditioning in fetuses. The unconditioned response to milk involves κ receptors (Smotherman & Robinson, 1992). Additionally, conditioning with milk and an artificial nipple in the rat fetus promotes κ and conditioned μ receptor activity (Robinson et al., 1993; Robinson & Smotherman, 1997). Classical conditioning of responses to an artificial nipple in the rat fetus employs both caudal κ receptor activity and rostral μ receptor activity (Petrov, Varlinskaya, & Smotherman, 2000). The role of opioids in conditioning in adults has been studied as well. Fanselow (1979) examined the effect of naloxone, another nonspecific opioid antagonist, on signaled-shock-preference conditioning to one side of a shuttlebox. Preference tests revealed that subjects that received naloxone during training did not show a subsequent side preference. Similarly, Foo and Helmstetter (2000) suggested the role of μ opioid receptors in mediating conditioned responses to shock in adult rats. Overall, results from these studies suggest a role of opioid receptors in behavioral conditioning and that multiple opioid receptors mediate the conditioning.
Since naltrexone is a nonspecific opioid antagonist, naltrexone may act at different receptors during the processes of acquisition (learning) and consolidation, offering a plausible explanation for the results from Experiments 2 and 3. For example, naltrexone may have blocked active κ receptors during the learning process, thus naltrexone-paired pups did not show conditioned behavioral activation as did the saline controls. Also, when given naltrexone after acquisition (using the example κ receptors were unaffected during the training), naltrexone may have then affected conditioned μ receptors during the process of consolidation of an odor preference. If μ receptors are involved in consolidating the memory of a stimulus as being rewarding, then blocking these receptors during consolidation would affect the number of choices made towards the odor when tested. If blocking these receptors during consolidation alters cellular activity in reward or aversion areas, the consolidation could be altered from a preference to aversion.
Further evidence for this interpretation was shown by Carr, Kutchukhidze, and Park (1999); systemic injections of naltrexone in adults induced c-fos immunoreactivity, which indicates increased cellular activity, in the extended amygdala. These areas—bed nucleus of the stria terminalis (BSTLD), nucleus accumbens shell (NACshell), and the central nucleus of the amygdala (ceA) —mediate motivation and reward. Carr et al. (1999) also showed that κ receptor blockade is responsible for the immunoreactivity in the BSTLD and CeA while μ receptor blockade is responsible for the increased activity in the NACshell. They concluded that the CeA might be under inhibitory control of both μ and κ receptors. These results suggest that blockade of receptors in particular areas of the reward pathway is responsible for our results. Cellular activation by naltrexone may alter perception of stimuli, which Park and Carr (1998) hypothesized to account for aversion and even suppression of the positive responses towards stimuli. Blocking κ receptors is not aversive (Carr, Papadouka, & Wolinsky, 1993; Leventhal, Kirkham, Cole, & Bodnar, 1995), thus if κ receptors participate in acquisition, this may explain why pups demonstrated neither an aversion nor preference in Experiment 2. If blockade of μ receptors is aversive (Shippenberg, 1993), and these receptors are involved in the consolidation of a conditioned odor preference, then this may explain why subjects demonstrated an aversion in Experiment 3; naltrexone altered opioid secretion in areas necessary for reward, thus altering the perception of the conditioned stimulus. Taken together, our results as well as results from the aforementioned studies suggest that odor conditioning involves several opioid receptors, and how and when developmentally they are altered may offer insight into the role of this neurotransmitter system in mother–infant attachment.
In conclusion, results from our study suggest a role of endogenous opioids in odor conditioning, with a critical, dual role during the sensitive period. Our results indicate that opioids function in both acquisition and consolidation of an odor preference. Endogenous opioids appear to be critical for preference formation to noxious stimuli that serve as reward, thus when the dam is somewhat abusive with the pups such as during rough transport, the pups will still associate her odor with good events (milk, warmth, and so on). To further understand the role of opioids in acquisition (learning) versus consolidation of olfactory preferences, receptor-specific drugs should be used; however, as demonstrated in this study, the mammalian model of odor–shock conditioning offers a valuable tool for investigation of their role.
Footnotes
Contract grant sponsor: NICHD
Contract grant number: 33402
Contract grant sponsor: HHS-PHS
Contract grant number: 1 F31 DA06082-01
The authors thank Dr. Donald Wilson for assistance in data analysis, and Drs. Victor Hutchison and Joseph Bastian for comments on an early draft of the manuscript. This work was funded by NICHD Grant 33402 to R. M. S. and HHS-PHS Grant 1 F31 DA06082-01 to T. L. R.
References
- Aroyewun O, Barr GA. Behavioral effects of opioid antagonists on milk intake of preweanling rats. Neuropharmacology. 1992;21:757–762. doi: 10.1016/0028-3908(82)90061-2. [DOI] [PubMed] [Google Scholar]
- Barr GA, Rossi G. Conditioned place preference from ventral tegmental injection of morphine in neonatal rats. Developmental Brain Research. 1992;66:133– 136. doi: 10.1016/0165-3806(92)90149-q. [DOI] [PubMed] [Google Scholar]
- Blass EM, Fitzgerald E. Milk-induced analgesia and comforting in 10-day-old rats: Opioid mediation. Pharmacology, Biochemistry and Behavior. 1988;29:9–13. doi: 10.1016/0091-3057(88)90266-3. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Woods PJ. The ontogeny of behavior in the albino rat. Animal Behavior. 1964;12:427–441. [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Developmental Psychobiology. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Carr KD, Kutchukhidze N, Park TH. Differential effects of μ and κ opioid antagonists on Fos-like immunoreactivity in extended amygdala. Brain Research. 1999;822:34–42. doi: 10.1016/s0006-8993(99)01088-4. [DOI] [PubMed] [Google Scholar]
- Carr KD, Papadouka V, Wolinsky TD. Norbinaltorphimine blocks the feeding but not the reinforcing effect of lateral hypothalamic electrical stimulation. Psychopharmacology. 1993;111:345–350. doi: 10.1007/BF02244951. [DOI] [PubMed] [Google Scholar]
- Clendeninn NJ, Petraitis M, Simon EJ. Ontological development of opiate receptors in rodent brain. Brain Research. 1976;118:157–160. doi: 10.1016/0006-8993(76)90852-0. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Scalzo FM, Enters EK, Spear N, Spear L. Effects of 6-hydroxydopamine-induced catecholamine depletion on shock-precipitated wall climbing of infant rat pups. Developmental Psychobiology. 1985;18:215–227. doi: 10.1002/dev.420180303. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Naloxone attenuates rat’s preference for signaled shock. Physiological Psychology. 1979;7:70–74. [Google Scholar]
- Foo H, Helmstetter FJ. Expression of antinociception in response to a signal for shock is blocked after selective downregulation of mu-opioid receptors in the rostral ventromedial medulla. Brain Research Molecular Brain Research. 2000;76:282–288. doi: 10.1016/s0169-328x(00)00009-7. [DOI] [PubMed] [Google Scholar]
- Hall WG. Feeding and behavioral activation in infant rats. Science. 1979;205:206–209. doi: 10.1126/science.451591. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Hall WG. Appetitive conditioning in neonatal rats: conditioned orientation to a novel odor. Developmental Psychobiology. 1982;15:379– 397. doi: 10.1002/dev.420150410. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Teicher M. Classical conditioning of an odor preference in 3-day-old rats. Behavioral and Neural Biology. 1980;29:132–136. doi: 10.1016/s0163-1047(80)92596-0. [DOI] [PubMed] [Google Scholar]
- Kehoe P. Opioid, behavior, and learning in mammalian development. In: Blass EM, editor. Behavioral neurobiology: Developmental psychobiology and behavioral ecology. Vol. 9. New York: Plenum Press; 1988. pp. 309–346. [Google Scholar]
- Kehoe P, Blass EM. Behaviorally functional opioid system in infant rats. I: Evidence for olfactory and gustatory classical conditioning. Behavioral Neuroscience. 1986;100:359–367. doi: 10.1037//0735-7044.100.3.359. [DOI] [PubMed] [Google Scholar]
- Kornblum HI, Hurlbut DE, Leslie FM. Postnatal development of multiple opioid receptors in the rat brain. Developmental Brain Research. 1987;37:21– 41. doi: 10.1016/0165-3806(87)90226-4. [DOI] [PubMed] [Google Scholar]
- Leventhal L, Kirkham TC, Cole JL, Bodnar RJ. Selective actions of central μ and κ opioid antagonists upon sucrose intake in sham-fed rats. Brain Research. 1995;685:205–210. doi: 10.1016/0006-8993(95)00385-4. [DOI] [PubMed] [Google Scholar]
- McLean JH, Darby-King A, Sullivan RM, King SR. Serotonergic influence on olfactory learning in the neonate rat. Behavioral and Neural Biology. 1993;60:152–162. doi: 10.1016/0163-1047(93)90257-i. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals (U. S. Public Health Service Publication No. 86–2. 3) Washington, DC: U. S. Government Printing Office; 1985. [Google Scholar]
- Okutani F, Yagi F, Kaba H. Gabaergic control of olfactory learning in young rats. Neuroscience. 1999;93:1297–1300. doi: 10.1016/s0306-4522(99)00224-9. [DOI] [PubMed] [Google Scholar]
- Park TH, Carr KD. Neuroanatomical patterns of fos-like immunoreactivity induced by a palatable meal and meal-paired environment in saline and naltrexone-treated rats. Brain Research. 1998;805:169–180. doi: 10.1016/s0006-8993(98)00719-7. [DOI] [PubMed] [Google Scholar]
- Pedersen PE, Williams CL, Blass EM. Activation and odor conditioning of suckling behavior in 3-day-old albino rats. Journal of Experimental Psychology. 1982;8:329–341. [PubMed] [Google Scholar]
- Petrillo P, Tavani A, Verotta D, Robson LE, Kosterlitz HW. Differential postnatal development of μ, δ, and κ, opioid binding sites in rat brain. Developmental Brain Research. 1987;31:53–58. doi: 10.1016/0165-3806(87)90082-4. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Becker LA, Smotherman WP. Endogenous mu opioid systems and suckling in the neonatal rat. Physiology & Behavior. 1998;65:591–599. doi: 10.1016/s0031-9384(98)00228-5. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Smotherman WP. Classical conditioning of responses to an artificial nipple in the rat fetus: Mu and kappa opioid systems. Developmental Psychobiology. 2000;37:59–72. doi: 10.1002/1098-2302(200009)37:2<59::aid-dev1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Price TL, Darby-King A, Harley CW, McLean JH. Serotonin plays a permissive role in conditioned olfactory learning induced by norepinephrine in the neonate rat. Behavioral Neuroscience. 1998;112:1430–1437. doi: 10.1037//0735-7044.112.6.1430. [DOI] [PubMed] [Google Scholar]
- Randall CK, Kraemer PJ, Dose JM, Carbary TJ, Bardo MT. The biphasic effect of morphine on odor conditioning in neonatal rats. Developmental Psychobiology. 1992;25:355–364. doi: 10.1002/dev.420250506. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Arnold HM, Spear NE, Smotherman WP. Experience with milk and an artificial nipple promotes conditioned opioid activity in the rat fetus. Developmental Psychobiology. 1993;26:375– 387. doi: 10.1002/dev.420260702. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Stimulus contingencies that permit classical conditioning of opioid activity in the rat fetus. Behavioral Neuroscience. 1997;111:1086 –1097. doi: 10.1037//0735-7044.111.5.1086. [DOI] [PubMed] [Google Scholar]
- Shide DJ, Blass EM. Opioid mediation of odor preferences induced by sugar and fat in 6-day-old rats. Physiology & Behavior. 1991;50:961–966. doi: 10.1016/0031-9384(91)90422-k. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS. Motivational effects of opioids. In: Herz A, editor. Handbook of experimental pharmacology: Opioids II. Vol. 104. New York: Springer-Verlag; 1993. pp. 633–650. [Google Scholar]
- Smotherman WP, Robinson SR. Kappa opioid mediation of fetal responses to milk. Behavioral Neuroscience. 1992;106:396–407. doi: 10.1037//0735-7044.106.2.396. [DOI] [PubMed] [Google Scholar]
- Spain JW, Roth BL, Coscia CJ. Differential ontogeny of multiple opioid receptors (μ, δ, and κ) Journal of Neuroscience. 1985;5:584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Plasticity in the reinforcement system of infant rats. Society for Neuroscience Abstracts. 1990;16:917. [Google Scholar]
- Sullivan RM, Wilson DA, Lemon C, Gerhardt GA. Bilateral 6-OHDA lesions of the locus coeruleus impair associative learning in newborn rats. Brain Research. 1994;643:306–309. doi: 10.1016/0006-8993(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Zyzak DR, Skierkowski P, Wilson DA. The role of olfactory bulb norepinephrine in early olfactory learning. Developmental Brain Research. 1992;70:279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- Tseng LF. The pharmacology of opioid peptides. U.S: Harwood Academic Publishers; 1995. [Google Scholar]
- Weldon DA, Fedorcik GG. Identification of the posttraining period when glutamate receptor blockade impairs olfactory learning in rat pups. Society for Neuroscience Abstracts. 1993;19:1010. [Google Scholar]
- Weldon DA, Travis ML, Kennedy DA. Post-training D1 receptor blockade impairs odor conditioning in neonatal rats. Behavioral Neuroscience. 1991;105:450–458. doi: 10.1037//0735-7044.105.3.450. [DOI] [PubMed] [Google Scholar]
- Woo CC, Leon M. Sensitive period for neural and behavioral response development to learned odors. Developmental Brain Research. 1987;36:309–313. doi: 10.1016/0165-3806(87)90038-1. [DOI] [PubMed] [Google Scholar]


