Abstract
In many altricial species, fear responses such as freezing do not emerge until sometime later in development. In infant rats, fear to natural predator odors emerges around postnatal day (PN) 10 when infant rats begin walking. The behavioral emergence of fear is correlated with two physiological events: functional emergence of the amygdala and increasing corticosterone (CORT) levels. Here, we hypothesize that increasing corticosterone levels influence amygdala activity to permit the emergence of fear expression. We assessed the relationship between fear expression (immobility similar to freezing), amygdala function (c-fos) and the level of corticosterone in pups in response to presentation of novel male odor (predator), littermate odor and no odor. CORT levels were increased in PN8 pups (no fear, normally low CORT) by exogenous CORT (3 mg/kg) and decreased in PN12 pups (express fear, CORT levels higher) through adrenalectomy and CORT replacement. Results showed that PN8 expression of fear to a predator odor and basolateral/lateral amygdala activity could be prematurely evoked with exogenous CORT, while adrenalectomy in PN12 pups prevented both fear expression and amygdala activation. These results suggest that low neonatal CORT level serves to protect pups from responding to fear inducing stimuli and attenuate amygdala activation. This suggests that alteration of the neonatal CORT system by environmental insults such as alcohol, stress and illegal drugs, may also alter the neonatal fear system and its underlying neural control.
Keywords: Corticosterone, Predator odor, Development, Infant rat, Fear, Amygdala, Hippocampus, Development
1. Introduction
Early adverse experience has been identified as a significant cause of adult behavioral problems, although very little is known about how these early experiences affect the infant and subsequent brain development (Glaser, 2000; Grossman et al., 2003; Gunnar, 2001; Sanchez et al., 2001; Teicher et al., 2003). It is well known that long-term adverse experience induces long-lasting changes in behavior and brain development, but a single exposure to a severe threat (such as taste aversion conditioning, predator presence) can also influence long-term changes in behavior and brain development (review in Wiedenmayer, 2004). Clinically, abused children show heightened fear and stress responses (Gunnar et al., 2001), although attenuated fear responses have also been documented (Gunnar, 2001; King et al., 2001). The cause of this clinical variability is unknown, although modification of the stress systems, the hypothalamus-pituitary-adrenal system that releases the stress hormone cortisol, and the other component of the stress axis composed of the locus coeruleus-amygdala has been implicated (Anand and Shekhar, 2003; Gunnar and Donzella, 2002; Kalin, 2003; Sanchez et al., 2001; Shekhar et al., 2003). Using an animal model, we began to assess the effects of early adverse experiences by presenting a naturally fearful odor (novel male odor) while manipulating the stress hormone corticosterone (CORT; homologous to primate cortisol) and assessed the early development of both the fear response and the neural structure implicated in fear responsiveness, the amygdala. We suggest that understanding the developmental neurobiology of infant fear responses will aid us in understanding how early adverse experiences alter brain development.
In altricial species, fear responses emerge later in development. For example, a delayed defensive response is seen in the young rabbit’s response to hawks (Pongrácz and Altbäcker, 2000) and stranger anxiety emerges around 9 months in children (Joseph, 1999). In rats, the novel adult male is a predator, but a defensive response to male odor does not emerge until approximately postnatal day (PN) 10, when pups begin to walk and sometimes leave the nest (Bolles and Woods, 1964; Bronstein and Hirsch, 1976; Brown, 1986; Mennella and Moltz, 1988; Paul and Kupferschmidt, 1975; Takahashi et al., 1991; Takahashi, 1992; Wiedenmayer and Barr, 2001). This PN10 early defensive response is characterized by an immature version of freezing sometimes referred to as immobility (Takahashi, 1994b), and corresponds with an increasing level of the stress hormone corticosterone, with later inclusion of fleeing (Wiedenmayer et al., 2003) and an analgesic response (Wiedenmayer and Barr, 1998) at weaning when the stress response to predator odor becomes more similar to the adult’s response (Dielenberg and McGregor, 2001; Perrot-Sinal et al., 1999).
The development of infant rat freezing to novel male odor and its associated neuroendocrine response has been characterized by Takahashi and his colleagues. They manipulated the age freezing emerged by increasing neonatal CORT levels (PN8). Specifically, premature freezing was elicited by increasing CORT levels, and freezing was eliminated by removing the primary source of CORT (adrenalectomy, ADX) in older pups (PN12; Takahashi and Rubin, 1993; Takahashi, 1994a,b). The ability of CORT to alter pups’ fear system is related to the stress hyporesponsive period (SHRP), when neonatal rats have very low basal levels of CORT and the CORT response to stressors (such as shock) is blunted (Grino et al., 1994; Levine, 1962, 2001; Walker et al., 1986). The SHRP blunted response to stress is believed to be due to tactile stimulation (licking and nursing) and/or feeding received during maternal care (Levine, 1962; Suchecki et al., 1993; Van Oers et al., 1998b).
As the SHRP begins to end, the immobility component of the PN10 fear response emerges along with amygdala’s participation in the fear response (Wiedenmayer and Barr, 2001). Specifically, during the SHRP, presentation of male odor does not elicit freezing and does not activate the amygdala, whereas a few days later the novel male odor elicits both freezing and amygdala participation (Wiedenmayer and Barr, 2001). Additional evidence suggests the hippocampus may also have a role in the development of freezing. CORT introduced directly into the hippocampus over 5 days accelerates cholinergic hippocampal development and the development of freezing (Takahashi, 1995; Takahashi and Goh, 1998). Others have found CORT (5 mg/kg from PN2 to PN6) to decrease hippocampus neurogenesis (Gould et al., 1991b,c; Gould and Cameron, 1997) suggesting CORT doses and regimen are important for hippocampal development during both infancy and adulthood (Cameron and Gould, 1994; Gould and Tanapat, 1999).
In the present study, we hypothesized that CORT is involved in the emergence of the fear response through the participation of the amygdala. It should be noted that CORT can be manipulated within the nest through the mother. First, mothers’ CORT levels are transmitted to pups through her milk, including the high CORT levels induced by stress (Yeh, 1984). Second, the amount of maternal sensory stimulation provided alters pups’ endogenous CORT levels, with high levels of stimulation maintaining low CORT levels and maternal deprivation increasing CORT levels (Dent et al., 2000, 2001; Levine et al., 1992; Suchecki et al., 1993). Third, pharmacological insults also alter pups’ CORT levels. Maternal ingestion of alcohol appears to prematurely end the SHRP (Weinberg, 1994) and opiates dampen infants’ CORT levels (Lesage et al., 1996, 1998). Therefore, our direct manipulations of pup CORT levels on fear expression and the amygdala may be related to brain and behavior alterations that occur normally during development.
2. Methods
2.1. Subjects
The subjects were 108 male and female PN8 and PN12 Long Evans rat pups born and bred at the University of Oklahoma’s vivarium (Harlan, Indianapolis, IN). The pups and their mothers were housed in polypropylene cages (34 cm × 29 cm × 17 cm) lined with aspen shavings and cages were kept in a temperature (23 °C) and light (07:00–19:00 h) controlled room. Food and water were available at all times. The day of birth was designated PN0, and litters were culled to 10 on PN1. Each experimental condition did not use more than one male and one female from each litter.
2.2. Corticosterone manipulation
For PN8 or PN12 pups with CORT replacement, pups were injected with either CORT (3.0 mg/kg, i.p.) or saline 30 min prior to odor presentations (Moriceau and Sullivan, 2004; Takahashi, 1994a). For PN12 pups, endogenous CORT was eliminated by ADX at PN8. Dorsal incisions to extract the adrenal glands were performed on anesthetized pups (isoflurane). SHAM-operated controls received dorsal incisions, but the adrenal glands were left intact. Following recovery from surgery (approximately 1 h), pups were returned to the mother until testing.
2.3. Odor presentations
Odors were presented to pups on either PN8 or PN12. Pups were placed in individual 600 ml glass beakers and given a 5 min adaptation period to recover from experimenter handling. They were then presented with the odor for 5 min. Either no odor, littermate, or adult male rat odor (rat pups had no prior experience with male odor) was delivered by a flow dilution olfactometer. Littermate and adult male odors were generated by placing each into separate round, airtight glass enclosures (width, 20.32 cm; height, 20.96 cm) connected to the olfactometer for odor delivery. Odor timing was controlled with a Chrontrol (Chrontol Corporation, San Diego, CA).
2.4. The total time immobile/freezing was recorded
It should be noted that pups do not show the entire spectrum of behaviors associated with freezing in the adult rat. For example, there is no piloerection and crouching position in PN12–14 pups, and immobile/freezing was defined as the cessation of body movement (Takahashi, 1994a,b; Wiedenmayer and Barr, 2001).
2.5. Immunohistochemistry
Ninety min following odor delivery, PN8 and PN12 pups were decapitated and their brains were quickly removed, frozen in 2-methylbutane at −45 °C and stored in a −70 °C freezer. For analysis, brains were sectioned in a cryostat (20 μm; Minotome Plus, TBS, Durham, NC) at −20 °C. Two out of every four sections were collected. Each third section was used for immunohistochemical processing (Fisher colorfrost/plus) and each fourth section was placed on a microscope slide for cresyl violet staining to permit localization of the amygdala and hippocampus. Sections of adult male-exposed and control animals were post-fixed and processed together. Sections were preincubated in hydrogen peroxide for 5 min. The sections were processed for 20 h at 4 °C in the primary antibody, rabbit anti-Fos (Santa Cruz Biotechnology, sc-52) diluted to 1:500 in phosphate buffer saline (pH 7.2). They were then rinsed and incubated in the secondary antibody, goat anti-rabbit (Vector Laboratories, Burlingame, CA) for 2 h at room temperature. Finally, the slides were processed using the ABC kit (Vectastain Elite, Vector Laboratories, Burlingame, CA). Stained sections were dehydrated in ethanol and coverslipped.
2.6. RIA
The levels of circulating CORT were determined from heart blood of PN8 and PN12 pups after 5 min exposure to littermate or male odor. Duplicate plasma samples were analyzed for CORT using the Rat corticosterone Coat-a-Countkit (Radioassay Systems Labs, In., Carson, CA). The sensitivity of the assay was 5 ng/ml. The intra-assay coefficient of variation was 1–9%.
2.7. Data analysis
Fos-positive cells were visualized using a microscope (Olympus with a 10× objective) equipped with a drawing tube. Using a rat brain atlas (Paxinos et al., 1991), cresyl violet sections were used to outline the basolateral/lateral complex, the medial nucleus and the central nucleus of the amygdala, as well as CA1, CA3 and the dentate gyrus of the hippocampus. Fos labeled cells were unilaterally counted by an experimenter blind to the training conditions. A Fos positive cell had to have a labeled nucleus to be considered distinct from the background. For each animal, the mean number of cell counts was calculated by averaging the counts from two sections. Comparisons of the number of Fos-positive cells were made with ANOVA and post hoc Fisher tests for each brain area. Freezing behavior was also analyzed with ANOVA followed by post hoc Fisher tests.
3. Results
3.1. PN8 behavior
As illustrated in Fig. 1A, saline treated PN8 pups did not exhibit immobility/freezing to any odor presentations, whereas CORT (3 mg/kg) injected pups showed a significant increase in immobility/freezing time to the adult male rat odor. ANOVA analysis revealed a significant interaction between odor presentation and drug treatment F(2,36) = 56.067, P < 0.0001; post hoc Fisher tests revealed that the CORT-male odor group was significantly different from each of the other PN8 groups at the P < 0.05 level. Minimal or no immobility/freezing was detected in response to either peer odor or the control no odor presentation. These results replicate previous work from other laboratories (Takahashi, 1994; Wiedenmayer and Barr, 2001).
Fig. 1.
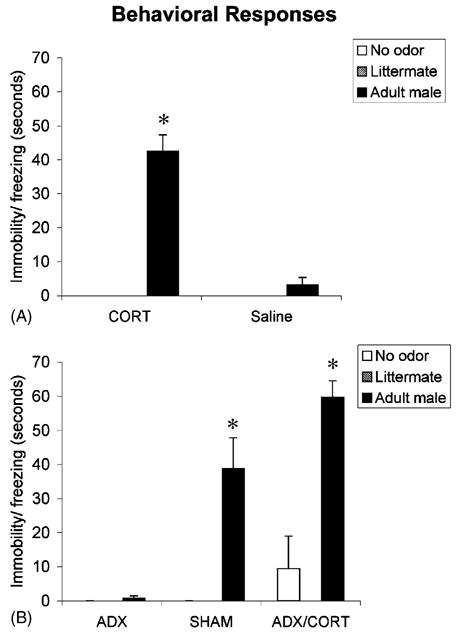
Mean (± S.E.M.) number of immobility/freezing responses for (A) PN8 and (B) PN12 pups to novel male odor, littermate odor or no odor. Some groups had values at or near zero and are not detectable on this graph. Asterisks represent significant differences from each of the other groups (P < 0.05).
3.2. PN8 hippocampus
No difference was found between groups in the CA1, CA3 or dentate gyrus of the hippocampus.
3.3. PN8 amygdala
A significant increase in Fos-positive cells was found in the basolateral/lateral complex of the amygdala of pups that received both the adult male rat odor and a CORT injection as compared to each of the other groups (Fig. 2A; ANOVA—odor presentation and drug, F(2,17) = 11.979, P < 0.001; post hoc Fisher tests revealed that the CORT-adult male odor group was significantly different from each of the other PN8 groups at the P < 0.05 level.). Minimal Fos-positive cells were found in the medial and central amygdala (nonsignificant ANOVA). This suggests that CORT either directly or indirectly permitted basolateral/lateral amygdala activation during the adult male odor presentation.
Fig. 2.
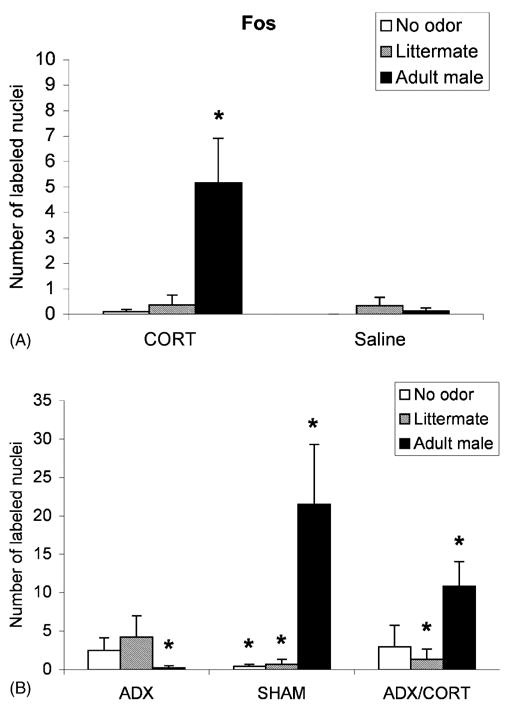
Mean (± S.E.M.) number of Fos-positive cells in the basolateral/lateral complex of the amygdala of rats exposed to an adult male odor, littermate odor or no odor for (A) PN8 and (B) PN12 pups. Some groups had values at or near zero and are not detectable on this graph. Asterisks represent significant differences from each of the other groups (P < 0.05).
3.4. PN8 and PN12 RIA
A significant increase in CORT level was found (ANOVA, F(3,8) = 6.345, P < 0.05) and post hoc Fisher tests (P < 0.05 level) revealed that PN12 pups exposed to male odor (56.67 ± 6.67 ng/ml) had significantly higher CORT levels compared to all other groups at both ages. PN12 pups exposed to littermate odor had a RIA value of 37.33 ± 6.67 ng/ml and PN8 RIA values for male odor and littermate odor were 27.33 ± 3.33 and 34.00 ± 0 ng/ml respectively.
3.5. PN12 behavior
As indicated in Fig. 1B, saline injected PN12 rats showed normal immobility/freezing to the adult male odor but immobility/freezing was eliminated in pups without CORT (ADX). However, replacement CORT in ADX pups (ADX/CORT) reinstated freezing in the presence of an adult male. These data replicate those of Takahashi (1994a,b) and suggest that CORT is required for the emergence of immobility/freezing during ontogeny. ANOVA analysis revealed a significant interaction between training condition and drug treatment F(4,45) = 10.330, P < 0.0001; post hoc Fisher tests (P < 0.05 level) revealed that the SHAM and ADX/CORT pups showed significantly more freezing to male odor compared to each of the other groups. Minimal or no freezing was detected in response to either littermate odor or the control no odor presentation.
3.6. PN12 hippocampus
No difference was found between groups in the CA1, CA3 or dentate gyrus of the hippocampus.
3.7. PN12 amygdala
As illustrated in Fig. 2B, both PN12 groups that showed freezing to male odor had a significant increase in Fos-positive cells in the basolateral/lateral complex of the amygdala in response to adult male odor than to no odor. It should be noted that the SHAM-male odor condition Fos expression was significantly higher than the ADX/CORT-male odor condition group. The Fos response was minimal in all other PN12 pups. ANOVA analysis revealed a significant interaction between training condition and drug treatment F(4,27) = 6.292, P < 0.05; post hoc Fisher tests revealed that the SHAM-male odor pups were significantly different from each of the other groups. The ADX/CORT male odor group was significantly different from each of the other groups, except the ADX-littermate group at the P < 0.05 level.
No group differences were found in the central nucleus of the amygdala or the medial nucleus of the amygdala.
4. Discussion
Our results suggest that the emergence of the stress CORT system permits the expression of fear (freezing) mediated by direct or indirect activation of the basolateral/lateral amygdala. Specifically, we could prematurely activate the expression of fear and the basolateral/lateral amygdala in pups during SHRP through exogenous CORT. We were also able to retard the developmental expression of fear and basolateral/lateral amygdala activation by preventing the end of the SHRP through ADX. These results replicate those of Wiedenmayer and Barr (2001) and Takahashi (1994a,b) but also extend their results to suggest that CORT may be implicated in the activation of the amygdala, either directly or indirectly, to permit the expression of infant fear. However, it should be noted that a longer exposure (30 min) to predator odor can increase CORT levels in younger pups (Tanapat et al., 1998) and could possibly activate the amygdala even in younger pups.
Our amygdala data, while consistent with other developmental analysis by Wiedenmayer and Barr (2001), are not consistent with the adult literature that suggests the medial nucleus of the amygdala, not the basolateral/lateral complex, is important in the response to predator odor (Dielenberg et al., 2001; Dielenberg and McGregor, 2001; Li et al., 2004). Our failure to show hippocampal participation in the predator odor response is also consistent with the work of Wiedenmayer and Barr (2001) but is also in sharp contrast to the adult literature (Heale et al., 1994; Mesches et al., 1999). It should be noted that Wiedenmayer and Barr (2001) found the inclusion of the hippocampus in the predator odor fear circuit at PN21 (weaning) and based on the learning literature, the hippocampus may not be functionally mature until weaning (Fanselow and Rudy, 1998; Green and Stanton, 1989; Rudy and Morledge, 1994). The reasons for developmental change in the predator odor circuitry is unclear and suggests the circuit may change with maturation and the inclusion of other fear related behaviors. For example, Wiedenmayer and Barr (2001) have shown that the medial nucleus of the amygdala does not become incorporated in the predator odor neural circuitry until around PN21 (weaning) when the fleeing response emerges.
While our data show a correlation between amygdala function and the expression of fear associated with systemic CORT manipulations, it is possible that CORT is working indirectly on the amygdala. Although the amygdala does contain CORT receptors during the SHRP and amygdala plasticity may be dependent upon CORT, other brain areas contain CORT receptors, connect with the amygdala, and are involved in fear such as the hippocampus, bed nucleus of the stria terminalis (BNST) and paraventricular nucleus of the hypothalamus (Cintra et al., 1993; Crain et al., 1979; Dielenberg and McGregor, 2001; Meaney et al., 1985; Nair and Gonzalez-Lima, 1999; Rosenfeld et al., 1988a,b, 1993; Sarrieau et al., 1988; Stutzmann et al., 1998). For example, prolonged CORT treatment over 4–5 days has been shown to accelerate the development of freezing and influence cholinergic hippocampal dentate granule cells’ development (Takahashi, 1995; Takahashi and Goh, 1998). However, due to the rapid action of CORT on the unlearned fear system in the present experiment (30 min), it is unlikely that neurogenesis could account for the precocial emergence of freezing in our PN8 pups. Perhaps the most cohesive assessment of neural development of pup freezing in response to predator odor was done by Wiedenmayer and Barr (2001), who showed that the basolateral amygdala, the locus coeruleus, the periaqueductal gray and the paraventricular nucleus of the hypothalamus each begin to participate in the response to a naturally fearful odor as freezing emerges. They also assessed weanling pups (PN21) and found the recruitment of the hippocampus, the BNST and the medial amygdala as the fleeing response emerges as a response to predator odor. Overall, the unlearned fear circuit appears complex and the data suggest that more than one circuit may be used for freezing and change over development.
We have found similar effects of CORT manipulation on the development of learned fear and participation of the amygdala in fear conditioning. Specifically, fear conditioning (odor 0.5 mA) in neonatal rat pups actually results in a preference for that odor (Camp and Rudy, 1988; Sullivan et al., 1986, 2000), although pups feel pain (Barr, 1995). In striking similarity to the unlearned fear system documented here, fear conditioning evoked odor aversion is not learned until around PN10, when the amygdala participates in odor-shock (0.5 mA) conditioning (Sullivan et al., 2000). Furthermore, we were able to alter the developmental expression of fear conditioning through manipulations of the CORT system similar to those described in the present experiments (Moriceau and Sullivan, 2004). Thus, both learned fear and unlearned fear appear to emerge at the same age, with the amygdala and CORT system implicated in both fear responses. Similarly to differences in amygdala participation found for predator odor, a more global response is found in the adult amygdala compared to the infant amygdala. Specifically, while our amygdala response was limited to the basolateral/lateral complex, the adult literature shows the basolateral/lateral complex, medial and central nuclei of the amygdala are activated by adult fear conditioning (Beane et al., 2002; Davis, 1997; Fanselow and LeDoux, 1999; Fanselow and Gale, 2003; Fendt and Fanselow, 1999; Goldstein et al., 1996; Johnson et al., 1992; Lee et al., 1994; Maren, 1999; Morrow et al., 2000; Roozendaal et al., 1991; Vazdarjanova et al., 2001; Walker and Davis, 1997). These differences may reflect the developmental changes in the fear circuitry.
These data suggest that modification of pups’ CORT system may modify the maturation of the fear system to predator odors. There are myriad ways pup CORT levels can be modified including through the mother’s milk (Yeh, 1984), the amount of maternal sensory stimulation (Dent et al., 2000, 2001; Levine et al., 1992; Suchecki et al., 1993) and maternal ingestion of alcohol (Weinberg, 1994) or opiates (Lesage et al., 1996, 1998). Indeed, the effects of stress and maternal behaviors that manipulate the CORT system appear to alter the trajectory of brain development (Brunson et al., 2001; Dent et al., 2000, 2001; Eghbal-Ahmadi et al., 1997; Francis et al., 2002; Huot et al., 2002; Smith et al., 1997; Zhang et al., 2002). Enhancing maternal behavior that maintains low CORT levels in pups reduces emotionality and enhances adult maternal behavior, while environmental stressors such as deprivation increase emotionality and produce a malfunctioning stress system. These early developmental manipulations of the infant’s CORT system produce long-term changes in baseline CORT levels, hormones and neurotransmitters related with the stress system and associated receptors in a myriad of brain structures (Avishai-Eliner et al., 1999; Caldji et al., 1998; Kent et al., 1997; Liu et al., 1997; Meaney et al., 1988, 1989, 1996; Okimoto et al., 2002; Rosenfeld et al., 1991; Rots et al., 1996; Stanton et al., 1988; Van Oers et al., 1998a; Vasquez et al., 1996; Yi et al., 1994).
Acknowledgments
This work was supported by grant NICHD-HD33402, NSF-IBN0117234 to RMS and OU Research Funds to SM, HHS-PHS NRSA F31 DA06082 to TLR and McNair Scholar funds to TO.
References
- Anand A, Shekhar A. Brain imaging studies in mood and anxiety disorders: special emphasis on the amygdala. Ann N Y Acad Sci. 2003;985:370–388. doi: 10.1111/j.1749-6632.2003.tb07095.x. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Hatalski CG, Tabachnik E, Eghbal-Ahmadi M, Baram TZ. Differential regulation of glucocorticoid receptor messenger RNA (GR-mRNA) by maternal deprivation in immature rat hypothalamus and limbic regions. Dev Brain Res. 1999;114:265–268. doi: 10.1016/s0165-3806(99)00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA. Ontogeny of nociception and antinociception. NIDA Res Monogr. 1995;158:172–201. [PubMed] [Google Scholar]
- Beane ML, Cole MA, Spencer RL, Rudy JW. Neonatal handling enhances contextual fear conditioning and alters corticosterone stress responses in young rats. Horm Behav. 2002;41:33–40. doi: 10.1006/hbeh.2001.1725. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Woods PJ. The ontogeny of behaviour in the albino rat. Anim Behav. 1964;12:427–441. [Google Scholar]
- Bronstein PM, Hirsch SM. Ontogeny of defensive reactions in Norway rats. J Comp Physiol Psychol. 1976;90:620–629. doi: 10.1037/h0077224. [DOI] [PubMed] [Google Scholar]
- Brown RE. Paternal behavior in the male Long–Evans rat. J Comp Psychol. 1986;100:162–172. [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci USA. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky P, Meaney M. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev Psychobiol. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Cintra A, Solfrini V, Bunnemann S, Okret S, Bortolotti F, Gustafsson JA, Fuxe K. Prenatal development of glucocorticoid receptor gene expression and immunoreactivity in the rat brain and pituitary gland: a combined in situ hybridization and immunocytochemical analysis. Neuroendocrinology. 1993;57:1133–1147. doi: 10.1159/000126480. [DOI] [PubMed] [Google Scholar]
- Crain B, Cotman C, Taylor D, Lynch G. A quantitative electron microscopic study of synaptogenesis in the dentate gyrus of the rat. Brain Res. 1979;63:195–204. doi: 10.1016/0006-8993(73)90088-7. [DOI] [PubMed] [Google Scholar]
- Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25:597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Dent GW, Okimoto DK, Smith MA, Levine S. Stress induced alterations in corticotropin-releasing hormone and vasopressin gene expression in the paraventricular nucleus during ontogeny. Neuroendocrinology. 2000;71:333–342. doi: 10.1159/000054554. [DOI] [PubMed] [Google Scholar]
- Dent GW, Smith MA, Levine S. Stress induced alterations in locus coeruleus gene expression during ontogeny. Dev Brain Res. 2001;127:23–30. doi: 10.1016/s0165-3806(01)00108-0. [DOI] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M, Hatalski CG, Avishai-Eliner S, Baram TZ. Corticotropin releasing factor receptor type II (CRF2) messenger ribonucleic acid levels in the hypothalamic ventromedial nucleus of the infant rat are reduced by maternal deprivation. Endocrinology. 1997;138:5048–5051. doi: 10.1210/endo.138.11.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Rudy JW. Convergence of experimental and developmental approaches to animal learning and memory processes. In: Carew TJ, Menzel R, Shatz CJ, editors. Mechanistic Relationships Between Development And Learning. Wiley; England: 1998. pp. 15–29. [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Glaser D. Child abuse and neglect and the brain: a review. J Child Psychol Psychiatry. 2000;41:7–116. [PubMed] [Google Scholar]
- Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol. 1991b;304:408–418. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell death. J Comp Neurol. 1991c;313:486–493. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA. Early NMDA receptor blockade impairs defensive behavior and increases cell proliferation in the dentate gyrus of developing rats. Behav Neurosci. 1997;111:49–56. doi: 10.1037//0735-7044.111.1.49. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Green RJ, Stanton ME. Differential ontogeny of working memory and reference memory in the rat. Behav Neurosci. 1989;103:98–105. doi: 10.1037//0735-7044.103.1.98. [DOI] [PubMed] [Google Scholar]
- Grino M, Paulmyer-Lacroix O, Faudon M, Renard M, Anglade G. Blockade of alpha2-adrenoreceptos stimulates basal and stress induced adrenocorticotropin secretion in the developing rat through a central mechanism independent from corticotropin-releasing factor and arginine vasopressin. Endocrinology. 1994;135:2549–2557. doi: 10.1210/endo.135.6.7988443. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Churchill JD, McKinney BC, Kodish IM, Otte SL, Greenough WT. Experience effects on brain development: possible contributions to psychopathology. J Child Psychol Psychiatry. 2003;44:33–63. doi: 10.1111/1469-7610.t01-1-00102. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Effects of early deprivation: findings from orphanage-reared infants and children. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. The Mit Press; 2001. pp. 17–38.pp. 617–629. [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev Psychopathol. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuoendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Heale VR, Vanderwolf CH, Kavaliers M. Components of weasel and fox odors elicit fast wave bursts in the dentate hyrus of rats. Behav Brain Res. 1994;63:159–165. doi: 10.1016/0166-4328(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neurosci Biobehav Rev. 1992;16:115–130. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- Joseph R. Environmental influences on neural plasticity, the limbic system, emotional developmental and attachment: a review. Child Psychiatry Hum Dev. 1999;29:189–208. doi: 10.1023/a:1022660923605. [DOI] [PubMed] [Google Scholar]
- Kalin NH. Nonhuman primate studies of fear, anxiety and temperament and the role of benzodiazepine receptors and the GABA system. J Clin Psychiatary. 2003;64:41–44. [PubMed] [Google Scholar]
- Kent S, Tom C, Levine S. Pituitary adrenal, feeding, and immune responses to interleukin 1-β in the neonate rat: interaction with maternal deprivation. Stress. 1997;1:213–231. doi: 10.3109/10253899709013742. [DOI] [PubMed] [Google Scholar]
- King JA, Mandansky D, King S, Fletcher HE, Brewer J. Early sexual abuse and low cortisol. Psychiatry Clin Neurosci. 2001;55:71–74. doi: 10.1046/j.1440-1819.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- Lee Y, Schulkin J, Davis M. Effect of corticosterone on the enhancement of the acoustic startle reflex by corticotropin releasing factor (CRF) Brain Res. 1994;666:93–98. doi: 10.1016/0006-8993(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Lesage J, Bernet F, Montel V, Dupuy JP. Effects of prenatal morphine on hypothalamic metabolism of neurotransmitters and gonadal and adrenal activities, during the early postnatalperiod in the rat. Neurochem Res. 1996;21:723–732. doi: 10.1007/BF02527731. [DOI] [PubMed] [Google Scholar]
- Lesage J, Grino M, Bernet F, Dutriez-Casteloot I, Montel V, Dupuy JP. Consequences of prenatal morphine exposure on the hypothalamo-pituitary-adrenal axis in the newborn rat: effects of maternal adrenalectomy. J Neuroendocrinol. 1998;10:331–342. [PubMed] [Google Scholar]
- Levine S. Plasma-free corticosterone response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- Levine S, Huchton DM, Wiener SG, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in infant rat. Dev Psychobiol. 1992;24:547–558. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiol Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Li C, Magliano TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav Neurosci. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky P, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors and hypothalamic pituitary adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Maren S. Long-term potentiation in the amygdala; a mechanism of emotional learning and memory. Trans Neurosci. 1999;22:561–567. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Sapolsky RM, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. II An autoradiographic study. Brain Res. 1985;350:165–168. doi: 10.1016/0165-3806(85)90260-3. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken D, Bhatnagar S, Van Berkel C, Sapolsky RM. Postnatal handling attenuates neuroendocrine, anatomical, and cognitive impairments related to the aged hippocampus. Science. 1988;238:766–768. [Google Scholar]
- Meaney MJ, Aitken D, Sharma S, Viau V, Sarrieau A. Postnatal handling increases hippocampal type II, glucocorticoid receptors and enhances adrenocortical negative feedback efficacy in the rat. Neuroendocrinology. 1989;51:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- Meaney M, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Moltz H. Infanticide in rats: male strategy and female counter-strategy. Physiol Behav. 1988;42:19–28. doi: 10.1016/0031-9384(88)90254-5. [DOI] [PubMed] [Google Scholar]
- Mesches MH, Fleshner M, Heman KL, Rose GM, Diamon DM. Exposing rats to a predator blocks primed burst potentiation in the hippocampus in vitro. J Neurosci. 1999;19:RC18. doi: 10.1523/JNEUROSCI.19-14-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Corticosterone influences on mammalian neonatal sensitive period learning. Behav Neurosci. 2004;118:274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Redmond AJ, Roth RH, Elsworth JD. The predator odor, TMT, displays a unique, stress-like pattern of dopaminergic and endocrinological activation in the rat. Brain Res. 2000;864:146–151. doi: 10.1016/s0006-8993(00)02174-0. [DOI] [PubMed] [Google Scholar]
- Nair HP, Gonzalez-Lima F. Extinction of behavior in infant rats: development of functional coupling between septal, hippocampal and ventral tegmental regions. J Neurosci. 1999;19:8646–8655. doi: 10.1523/JNEUROSCI.19-19-08646.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okimoto DK, Blaus A, Schmidt M, Gordon MK, Dent GW, Levine S. Differential expression of c-fos and tyrosine hydroxylase mRNA in the adrenal gland of the infant rat: evidence for an adrenal hyporesponsive response period. Endocrinology. 2002;143:1717–1725. doi: 10.1210/endo.143.5.8819. [DOI] [PubMed] [Google Scholar]
- Paul L, Kupferschmidt J. Killing of conspecifc and mouse young by male rats. J Comp Physiol Psychol. 1975;88:755–763. [Google Scholar]
- Paxinos G, Tork I, Tecott LH, Valentino KL. Atlas of the Developing Rat Brain. Academic Press; United Kingdom Edition: 1991. [Google Scholar]
- Perrot-Sinal TS, Ossenkopp KP, Kavaliers M. Brief predator odour exposure activates the HPA axis independent of locomotor changes. Neuroreport. 1999;10:775–780. doi: 10.1097/00001756-199903170-00021. [DOI] [PubMed] [Google Scholar]
- Pongrácz P, Altbäcker V. Ontogeny of the responses of European rabbits (Oryctolagus cuniculus) to aerial and ground predators. Can J Zool. 2000;78:655–665. [Google Scholar]
- Roozendaal B, Koolhass JM, Bohus B. Attenuated cardiovascular, neuroendocrine, and behavioral responses after a single footshock in central amygdaloid lesioned male rats. Physiol Behav. 1991;50:771–775. doi: 10.1016/0031-9384(91)90016-h. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Sutanto W, Levine S, De Kloet ER. Ontogeny of type I and type II corticosteroid receptors in the rat hippocampus. Dev Brain Res. 1988a;42:113–118. doi: 10.1016/0165-3806(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Van Eekelen JAM, Levine S, De Kloet ER. Ontogeny of the type II glucocorticoid receptors in discrete rat brain regions: an immunocytochemical study. Dev Brain Res. 1988b;42:119–127. doi: 10.1016/0165-3806(88)90207-6. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Guttierrez YA, Martin AM, Mallet HA, Alleva E, Levine S. Maternal regulation of the adrenocortical response in preweanling rats. Physiol Behav. 1991;50:661–671. doi: 10.1016/0031-9384(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Van Eekelen JAM, Levine S, De Kloet ER. Ontogeny of corticosteroid receptors in the brain. Cell Mol Neurobiol. 1993;13:295–319. doi: 10.1007/BF00711575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rots NY, De Jong J, Workel JO, Levine S, Cools AR, De Koet ER. Neonatal maternally deprived rats have as adults elevated basal pituitary adrenal activity and enhanced susceptibility to apomorphine. J Neuroendocrinol. 1996;8:501–506. doi: 10.1046/j.1365-2826.1996.04843.x. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. The ontogeny of contextual fear conditioning: implications for consolidation infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–450. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sarrieau A, Sharma S, Meany MJ. Postnatal development and environmental regulation of hippocampal glucocorticoid and mineralocorticoid receptors. Dev Brain Res. 1988;43:158–162. doi: 10.1016/0165-3806(88)90162-9. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. N Y Acad Sci. 2003;985:308–325. doi: 10.1111/j.1749-6632.2003.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Kim SY, Van Oers HJJ, Levine S. Maternal deprivation and stress induce immediately early genes in the infant rat brain. Endocrinology. 1997;138:4622–4628. doi: 10.1210/endo.138.11.5529. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behav Neurosci. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- Stutzmann G, McEwen B, LeDoux J. Serotonin modulation of sensory inputs to the lateral amygdala: dependency on corticosterone. J Neurosci. 1998;18:9529–9538. doi: 10.1523/JNEUROSCI.18-22-09529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchecki D, Mozaffarian D, Gross G, Rosenfeld P, Levine S. Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology. 1993;57:204–212. doi: 10.1159/000126361. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Dev Psychobiol. 1986;19:615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy: ontogeny of conditioned fear and the amygdala. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK, Turner JG, Kalin NH. Development of stress induced responses in preweanling rats. Dev Psychobiol. 1991;24:341–360. doi: 10.1002/dev.420240504. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Ontogeny of behavioral inhibition induced by unfamiliar adult male conspecifics in pre-weanling rats. Physiol Behav. 1992;52:493–498. doi: 10.1016/0031-9384(92)90336-z. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Rubin WW. Corticosteroid induction of threat induced behavioral inhibition in preweanling rats. Behav Neurosci. 1993;107:860–866. doi: 10.1037//0735-7044.107.5.860. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Organizing action of corticosterone on the development of behavioral inhibition in the preweanling rat. Dev Brain Res. 1994a;81:121–127. doi: 10.1016/0165-3806(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Stimulus control of behavioral inhibition in the preweanling rat. Physiol Behav. 1994b;55:717–721. doi: 10.1016/0031-9384(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Glucocorticoids, the hippocampus, and behavioral inhibition in the preweanling rat. J Neurosci. 1995;15:6023–6034. doi: 10.1523/JNEUROSCI.15-09-06023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK, Goh CS. Glucocorticoid facilitation of cholinergic development in the rat hippocampus. Neuroscience. 1998;83:1145–1153. doi: 10.1016/s0306-4522(97)00472-7. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Galea LAM, Gould E. Stress inhibits the proliferation of granule cell percursors in the developing dentate gyrus. Int J Dev Neurosci. 1998;16:235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersena SL, Polcarib A, Andersena CM, Navaltae CP, Kima DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Van Oers HJJ, de Kloet ER, Li C, Levine S. The ontogeny of glucocorticoid negative feedback: influence of maternal deprivation. Endocrinology. 1998a;139:2838–2846. doi: 10.1210/endo.139.6.6037. [DOI] [PubMed] [Google Scholar]
- Van Oers HJJ, de Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but by suppressing corticosterone. J Neurosci. 1998b;18:10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Cahill L, McGaugh JL. Disrupting basolateral amygdala function impairs unconditioned freezing and avoidance in rats. Eur J Neurosci. 2001;14:709–718. doi: 10.1046/j.0953-816x.2001.01696.x. [DOI] [PubMed] [Google Scholar]
- Vasquez DM, Van Oers HJJ, Levine S, Akil H. Regulation of glucocorticoid and mineralocorticoid receptor mRNAs in the hippocampus of the maternally deprived infant rats. Brain Res. 1996;731:79–90. doi: 10.1016/0006-8993(96)00465-9. [DOI] [PubMed] [Google Scholar]
- Walker CD, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. Recent studies on the effects of fetal alcohol exposure on the endocrine and immune system. Alcohol Alcohol. 1994;2:401–409. [PubMed] [Google Scholar]
- Wiedenmayer CP, Barr GA. Ontogeny of defensive behavior and analgesia in rat pups exposed to an adult male rat. Physiol Behav. 1998;63:261–269. doi: 10.1016/s0031-9384(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Barr GA. Developmental changes in c-fos expression to an age-specific social stressor in infant rats. Behav Brain Res. 2001;126:147–157. doi: 10.1016/s0166-4328(01)00260-1. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Magarinos AM, McEwen BS, Barr GA. Mother lowers glucocorticoid levels of preweanling rats after acute threat. Ann N Y Acad Sci. 2003;1008:304–307. doi: 10.1196/annals.1301.038. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP. Adaptations or pathologies? Long-term changes in brain and behavior after a single exposure to severe threat. Neurosci Biobehav Rev. 2004;28:1–12. doi: 10.1016/j.neubiorev.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Yeh KY. Corticosterone concentration in the serum and milk of lactating rats: parallel changes after induced stress. Endocrinology. 1984;115:1364–1370. doi: 10.1210/endo-115-4-1364. [DOI] [PubMed] [Google Scholar]
- Yi SJ, Masters JN, Baram TZ. Glucocorticoid receptor mRNA ontogeny in the fetal and postnatal rat forebrain. Mol Cell Neurosci. 1994;5:385–393. doi: 10.1006/mcne.1994.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LX, Levine S, Dent G, Zhan Y, Xing G, Okimoto D, Gordon MK, Post RM, Smith MA. Maternal deprivation increases cell death in the infant rat brain. Dev Brain Res. 2002;133:1–11. doi: 10.1016/s0926-6410(01)00118-5. [DOI] [PubMed] [Google Scholar]


