Abstract
Background
For many women, pregnancy begets long-term weight gain. Modifiable behaviors that contribute to postpartum weight retention have not been well studied.
Methods
Prospective cohort study of 902 women enrolled in Project Viva, examining associations of postpartum television viewing, walking, and trans fat intake with weight retention ≥5kg at 12 months postpartum. Data were collected in 1999–2003 and analyzed in 2005–2006.
Results
At 6 months postpartum, women reported a mean (SD) of 1.7 (1.3) hours of television viewing, 0.7 (0.7) hours of walking, and 1.1% (0.5) of energy intake from trans fat per day. At 1 year, participants retained a mean of 0.6 kg (range: −17.3 to 25.5), and 12% retained at least 5kg. In multivariate logistic regression models, adjusting for maternal sociodemographics, parity, pre-pregnancy body mass index, gestational weight gain, breastfeeding, and smoking, the odds ratio of retaining at least 5kg was 1.24 (95% confidence interval [CI]: 1.06–1.46) per daily hour of television viewing, 0.66 (95% CI: 0.46–0.94) per daily hour of walking, and 1.33 (95% CI: 1.09–1.62) per 0.5% increment in daily energy intake from trans fat. Women who watched less than 2 hours of television, walked at least 30 minutes, and consumed trans fat below the median had an odds ratio of 0.23 (95% CI: 0.08–0.66) of retaining at least 5 kg.
Conclusions
Postpartum television viewing, walking, and trans fat intake were associated with weight retention. Interventions to modify these behaviors may help reduce excess postpartum weight gain and prevent obesity among women.
INTRODUCTION
The puerperal period may represent a critical window for long-term weight gain and the development of obesity.1,2 Compared with weight gain during other periods of life, excess weight retained after giving birth appears to be particularly harmful, as evidence suggests that it is deposited preferentially in central rather than peripheral sites.3,4
Postpartum weight retention is defined as the difference between weight at some time after delivery and weight prior to pregnancy. Previous studies indicate that mean postpartum weight retention is approximately 0.5 to 1 kg at 12 months, with a wide range of weight changes from more than 10 kg lost to more than 20 kg gained.5 Despite this modest mean change, a large subset of women have substantial weight retention.6 The reported proportion of women retaining 5kg or more 6 to 12 months postpartum has ranged from 14% to 25%.6,7
Gestational weight gain is the strongest predictor of weight retention following pregnancy.2,6 Other identified risk factors, including nonwhite race/ethnicity, primiparity, and high pre-pregnancy weight, are not modifiable or occur prior to pregnancy. Diet, physical activity, and inactivity provide three major behavioral targets for optimizing energy balance. Among nonpregnant adults, a large number of studies have examined modifiable lifestyle determinants of weight gain, yet few investigators have examined these factors during the postpartum period.2 In a recent analysis, Olson et al.8 reported that women who ate “much less food” 6 to 12 months postpartum than 0 to 6 months, and those who exercised “often,” were less likely to retain at least 4.55kg (10 pounds) at 1 year. Limitations of that and other studies 5,6,9,10 include behavior measures that are difficult to translate into specific recommendations, no assessment of inactivity, and use of diet quantity, which may be more difficult to modify than diet quality.11 The present study examines associations of television viewing, physical activity (especially walking), and dietary factors including intake of fiber, total fat, and trans fat in the early postpartum period with substantial weight retention at 1 year postpartum. These behaviors were selected a priori because they are associated with weight gain and obesity-related disease risk among men and nonpregnant women,12–16 and are likely to be modifiable.
METHODS
Population and Study Design
Women attending their initial prenatal visit at one of eight urban and suburban obstetrical offices in a multi-specialty group practice in eastern Massachusetts were recruited.17,18 Eligibility criteria included fluency in English, gestational age <22 weeks, and singleton pregnancy; 65% eligible women were recruited. All women provided informed consent, and all procedures were approved by a human studies committee and in accordance with ethical standards for human experimentation.19 This study includes data collected in 1999–2003, and analyzed in 2005–2006.
Of the 2128 participating women who gave birth, 1585 enrolled for study continuation beyond 6 months postpartum. Information on both pre-pregnancy and 1-year postpartum weight as well as behaviors at 6 months postpartum was available from 1092 women. Women who became pregnant in the first year postpartum (n =102), missing information on height, race/ethnicity, parity, education, smoking, physical activity, marital, or work status (n =72), and who reported implausible amounts of physical activity (n =6) (walking ≥5 hrs/day, light/moderate activity ≥3 hrs/day, or vigorous activity ≥3 hrs/day) were excluded. The 902 women (57% of 1585) included in this analysis were somewhat more likely to be white (79% vs 70%) and had slight differences in mean pre-pregnancy body mass index (BMI) (24.3 vs 24.9 kg/m2), gestational weight gain (15.6 vs 15.4 kg), walking time (0.74 vs 0.80 hours daily), and daily trans fat intake (1.1 vs 1.2% of daily total energy), but did not differ in television viewing (1.7 vs 1.7 hours daily), compared with the 1585 women potentially eligible.
Main Exposures—Television Viewing, Physical Activity, and Diet—At 6 Months Postpartum
At 6 months postpartum, participants reported the average weekly hours they spent watching television or videos and in leisure-time physical activity. Physical activity was classified as walking (“for fun or exercise, including to or from work, but not at work”), light/moderate physical activity (“such as yoga, bowling, stretching classes, and skating, not including walking”), and vigorous recreational activities (“such as jogging, swimming, cycling, aerobic class, skiing, or other similar activities”). These questions were derived from the Physical Activity Scale for the Elderly (PASE),20 but instead of using the previous 7 days, women averaged their activity over the previous month, and reported average hours per week rather than both days per week and hours per day. Light and moderate activities were combined. The choice of examples of activities was influenced by the PASE, the Paffenbarger physical activity questionnaire,21 and knowledge of activities common to women in the northeastern U.S. Walking was of primary interest as a measure of physical activity, because of results from Project Viva that time spent walking did not decrease from pre-pregnancy to the postpartum period, whereas both light/moderate and vigorous physical activity did.22 Also at 6 months postpartum, participants completed a brief food frequency questionnaire called PrimeScreen, which included 21 questions about intake of foods and food groups since delivery, including questions quantifying intake of stick margarine, baked products, and deep-fried foods.23 PrimeScreen is reproducible and comparable to estimates of intake from a validated full-length food frequency questionnaire for a number of nutrients including energy-adjusted fiber (r =0.68 for reproducibility over time and r=0.58 for comparability with full length dietary questionnaire), trans fatty acids (r=0.80 and r=0.64), and saturated fat (r =0.76 and r =0.59).23 The Harvard nutrient database, which has been used for several large cohort studies,24 was used to calculate daily intake of nutrients. Intake of fats was adjusted for total energy intake using nutrient density, and nutrient residuals otherwise.11
Postpartum Weight Retention
Women reported their weight on a mailed questionnaire at 12 months postpartum. Weight retention was calculated as the difference between the self reported postpartum and pre-pregnancy weights. In a validation study, self-reported pre-pregnancy weight was compared with clinically measured weights among 170 participants who had a weight recorded in the medical record within 3 months prior to their last menstrual period. The association between self-reported and clinically measured weight was linear. Correlation coefficients (r= 0.99 overall) and mean underreporting of weight (approximately 1 kg overall) did not differ by maternal race/ethnicity, gestational age at enrollment into the study, or weight itself. The very high correlation indicates that ranking of individuals is well preserved. Although a similar validation was not performed postpartum, the magnitude of any underreporting is likely to have been similar at both time points.
Covariates
Maternal age, race/ethnicity, parity, education, and household income were included in the analysis as covariates, all reported by the women at their first study visit. Pre-pregnancy BMI (kg/m2) was calculated from self-reported height and weight. Pregnancy weights were obtained from the medical record. Pregnancy weight gain was calculated as the difference between the last weight prior to delivery and the self-reported pre-pregnancy weight, and classified as inadequate, adequate, or excessive.25 At 6 months postpartum, women reported smoking habits and employment and completed an Edinburgh postpartum depression questionnaire.26
Data Analysis
Associations of participant characteristics with postpartum weight retention were evaluated using t tests, Wilcoxon rank sum, and chi square analyses. Independent effects of 6 month postpartum television viewing, physical activity, and diet on weight retention at 12 months postpartum were studied using multivariable logistic regression. Individual nutrients studied were fiber, glycemic index, total fat, saturated fat, and trans fat. Glycemic index and saturated fat intake were not associated with weight retention. Because intake of fiber, total fat, and trans fat were strongly correlated with each other, each nutrient was evaluated individually and then in combination with the others. Trans fat was studied most extensively, as it was associated with weight retention independent of the other nutrients.
Separate analyses were performed for television viewing, walking, and trans fat intake, and then these three primary predictors were included together in the same model. Each primary exposure was examined as a continuous variable, and in a secondary analysis in quartiles. Covariates were considered based on the review of the literature and included if they were independent predictors of weight retention or confounded associations of behaviors with postpartum weight retention. Included covariates were maternal sociodemographic factors, parity, pre-pregnancy BMI, gestational weight gain, breastfeeding, employment, and smoking. Gestation length was not included as it did not influence results. Postpartum depression, light/moderate and vigorous activity and the time between delivery and reporting of 1-year postpartum weight were investigated to determine whether they altered effect estimates for exposures of interest. Effect modification by parity (0, ≥1), race/ethnicity (white, nonwhite), and pre-pregnancy BMI (< or ≥25 kg/m2) were investigated using interaction terms and stratification.
Additionally, beneficial exposure categories were defined as trans fat intake < median, watching <2 hours of television daily, and walking ≥30 minutes daily. Women reporting 3, 2 or 1 beneficial behaviors were compared with those reporting no beneficial behaviors, adjusting for the same covariates as in the main analysis. All analyses were performed using SAS version 8.2 (SAS institute, Cary NC).
RESULTS
Participating women were 21% nonwhite, including 8% black and 5% Hispanic; 76% had graduated from college. Mean (SD) age was 33.0 (4.7) years and pre-pregnancy BMI 24.3 (4.8) kg/m2. At 6 months postpartum, women reported a mean (SD) of 1.7 (1.3) hours of television viewing, 0.7 (0.7) hours of walking, 0.2 (0.3) hours of moderate physical activity, and 0.2 (0.3) hours of vigorous physical activity per day. Mean (SD) reported daily intake of total fat was 30.7% (7.2) of total energy, and intake of trans fat was 1.1% (0.5) of total energy.
Mean weight retention was 0.6 kg, with a range of −17.3 to 25.5 kg; 111 mothers (12%) retained at least 5 kg. Time between delivery and reporting of 1-year postpartum weight (mean 13.2 months) was not associated with the amount of weight retained. Mothers who retained at least 5 kg were younger, heavier before pregnancy, more likely to be nonwhite, unmarried, primiparous, and have lower income, and to have gained an excessive amount of weight during pregnancy. At 6 months postpartum they reported more hours viewing television and more intake of trans fat (Table 1). In these unadjusted results, postpartum walking, light/moderate activity, vigorous activity, return to work, breastfeeding, rates of depression, and smoking habits did not differ (Table 1). Compared with pre-pregnancy, television viewing did not change (mean [SD] difference 0.05 [1.23] hours/day).22
Table 1.
Demographic characteristics and associations with weight retention at 1 year postpartum among women in Project Viva.
| Weight retention at 1 year postpartum
|
|||
|---|---|---|---|
| Characteristic | < 5 kg n =791 (88%)
|
≥ 5 kg n =111 (12%)
|
p valuea |
| Mean (SD) or percent | |||
| Pre-pregnancy | |||
| Age (years) | 33.2 (4.4) | 31.5 (6.2) | 0.007 |
| Body mass index (kg/m2) | 24.2 (4.8) | 25.6 (4.8) | 0.003 |
| Race/ethnicity | <0.001 | ||
| White | 81 | 63 | |
| Black | 6 | 19 | |
| Hispanic | 5 | 6 | |
| Other | 7 | 12 | |
| Education | <0.001 | ||
| Some college or less | 21 | 39 | |
| College graduate | 39 | 44 | |
| Graduate degree | 40 | 17 | |
| Household income | <0.001 | ||
| Don’t know or missing | 4 | 11 | |
| ≤ $40,000 | 9 | 15 | |
| $40,001–$70,000 | 20 | 24 | |
| >$70,000 | 68 | 50 | |
| Parity: | 0.02 | ||
| 0 previous births | 46 | 58 | |
| ≥ 1 previous births | 54 | 42 | |
| Marital status | <0.001 | ||
| Married or cohabitating | 97 | 85 | |
| Single | 3 | 15 | |
| During pregnancy | |||
| Gestational weight gain | <0.001 | ||
| Excessive | 46 | 76 | |
| Adequate | 38 | 21 | |
| Inadequate | 15 | 4 | |
| 6-months postpartum | |||
| Television viewing (hours/day) | 1.6 (1.3) | 2.1 (1.5) | <0.001 |
| Television viewing <2 hours/day | 68 | 54 | 0.003 |
| Walking (hours/day) | 0.74 (0.66) | 0.73 (0.69) | 0.82 |
| Walking ≥ 30 minutes/day | 57 | 52 | 0.36 |
| Moderate physical activity (hr/day) | 0.16 (0.29) | 0.23 (0.48) | 0.49 |
| Any moderate activity | 39 | 41 | 0.75 |
| Vigorous physical activity (hr/day) | 0.16 (0.29) | 0.19 (0.41) | 0.61 |
| Any vigorous activity | 36 | 31 | 0.31 |
| Trans fat intake (% of energy) | 1.1 (0.5) | 1.3 (0.6) | <0.001 |
| Trans fat intake < median | 52 | 32 | <0.001 |
| Total fat intake (% of energy) | 30.3 (7.0) | 33.5 (7.3) | <0.001 |
| Fiber intake (grams/day) | 8.6 (3.0) | 7.4 (2.8) | <0.001 |
| Employment status | 0.75 | ||
| Working at a paying job | 69 | 70 | |
| Not working at a paying job | 31 | 30 | |
| Smoking | 0.51 | ||
| Yes | 5 | 4 | |
| No | 95 | 96 | |
| Edinburgh depression scale score | 0.09 | ||
| <13 | 93 | 87 | |
| 13–14 | 3 | 6 | |
| ≥15 | 4 | 6 | |
| Breastfeeding status | 0.38 | ||
| Formula only, never breastfed | 10 | 10 | |
| Weaned from breast to formula | 36 | 44 | |
| Mixed formula and breast milk | 27 | 23 | |
| Exclusively breastfeeding | 27 | 23 | |
p value from t tests (age, body mass index), Wilcoxon rank sum (television viewing, physical activity, and diet), or chi square analyses (remaining variables).
SD, standard deviation
For each daily hour of television viewing, the adjusted odds ratio (OR) for retaining at least 5kg was 1.24 (95% confidence interval [CI] 1.06–1.46). Trans fat intake similarly increased risk (adjusted OR 1.33, 95% CI = 1.09, 1.62 for each 0.5% of energy from trans fat; Table 2). Adjustment for depression, light/moderate and vigorous physical activity did not alter the detrimental effects of television viewing or trans fat intake (Table 2).
Table 2.
Adjusted odds ratios of women retaining at least 5 kg at 1 year postpartum.
| Odds of retaining at least 5 kg at 1 year postpartum
|
||||||
|---|---|---|---|---|---|---|
| Per daily hour of television viewing
|
Per daily hour of walking
|
Per 0.5% of energy from
|
||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Model 1 | 1.24 | 1.09, 1.42 | 0.89 | 0.65, 1.22 | 1.35 | 1.14, 160 |
|
| ||||||
| Model 2 | 1.16 | 1.01, 1.33 | 0.74 | 0.54, 1.03 | 1.23 | 1.02, 1.48 |
|
| ||||||
| Model 3 | 1.19 | 1.03, 1.39 | 0.73 | 0.51, 1.03 | 1.34 | 1.10, 1.64 |
|
| ||||||
| Model 4 | 1.24 | 1.06, 1.46 | 0.66 | 0.46, 0.94 | 1.33 | 1.09, 1.62 |
|
| ||||||
| Model 5 | 1.26 | 1.07, 1.48 | 0.59 | 0.40, 0.88 | 1.32 | 1.08, 1.62 |
Model 1 = adjusted for age.
Model 2 = Model 1 + education (< college, college graduate, graduate degree), race/ethnicity (black, Hispanic, white, other), household income (≤ $40,000, $40,001–$70,000, > $70,000, missing), marital status (single, married/cohabitating), and postpartum employment (not working, working at a paying job)
Model 3 = Model 2 + parity (0, 1+), pre-pregnancy body mass index (continuous), weight gain during pregnancy (inadequate, adequate, excessive), breastfeeding status at 6 months (formula only, weaned, mixed feeding, exclusive breastfeeding), postpartum smoking (yes, no)
Model 4 = Model 3 + includes all 3 main exposures (television viewing, walking, and trans fat intake).
Model 5 = Model 4 + depression, light/moderate and vigorous physical activity
CI, confidence interval; OR, odds ratio
Trans fat intake was correlated with intake of both fiber (Spearman r = −0.55) and total fat (Spearman r = 0.77). Daily intake of fiber and total fat were each associated with postpartum weight retention ≥5 kg, although neither association remained after including both nutrients. For example, the adjusted odds ratio for fiber (0.72, 95% CI = 0.56, 0.94 per 3g/day), was reduced (0.87, 95% CI = 0.63, 1.20) after adjustment for total fat intake. Similarly, the effect of total fat (OR 1.64, 95% CI = 1.18, 2.30 per 10% of energy from fat) was reduced (OR 1.46, 95% CI = 0.96, 2.23) after adjustment for fiber intake. Additional adjustment for physical activity did not alter estimates for fiber or total fat (data not shown). After adjustment for fiber intake, trans fat remained associated with substantial weight retention (OR 1.26, 95% CI = 1.02, 1.56 per 0.5% of energy from trans fat). Because of the high correlation between trans and total fat, including both nutrients in the same model resulted in substantially wider confidence intervals for both.
Walking did not appear to be associated with weight retention on bivariate analysis and after adjustment for age (Table 2). An association of walking with weight retention was found after adjustment for participant characteristics and television viewing (OR 0.66, 95% CI = 0.46–0.95), with little additional change after inclusion of trans fat intake (Table 2). Walking appeared even more beneficial after adjustment for light/moderate and vigorous activity (Model 5, Table 2). In this model neither light/moderate (OR 1.53, 95% CI = 0.77–3.03 per daily hour) nor vigorous activity (OR 1.06, 95% CI = 0.96–1.17 per daily hour) was associated with retaining ≥5kg, perhaps because only 353 (39%) women reported any light/moderate activity and 315 (35%) any vigorous activity. The effect of walking was also substantial when the analysis was restricted to the 441 participants who reported no postpartum light/moderate or vigorous activity (OR 0.47, 95% CI = 0.25–0.88 per daily hour of walking). There was no modification of the effects of television viewing, walking, and trans fat intake by race/ethnicity, parity, or pre-pregnancy BMI (all p values for interaction terms >0.15).
When additionally included, pre-pregnancy television viewing and physical activity were not associated with weight retention, and did not alter the observed significant effects of the three postpartum behaviors (data not shown). When postpartum behaviors were evaluated in categories rather than as continuous variables, associations with postpartum weight retention were generally linear. Adjusted odds ratios for the 4th through 2nd quartiles, each compared with the lowest quartile (OR 1.0) were 0.64 (95% CI = 0.35–1.17), 0.90 (95% CI = 0.47–1.73), and 1.02 (95% CI = 0.55–1.88) for walking; 2.21 (95% CI = 1.14–4.29), 1.57 (95% CI = 0.81–3.05), and 1.13 (95% CI = 0.50–2.52) for television viewing; and 1.92, (95% CI = 1.01–3.66), 1.25 (95% CI = 0.65–2.41), and 0.70 (95% CI = 0.34–1.43) for trans fat intake.
Beneficial categories of the three behaviors were defined as trans fat intake < median (1.06% of total energy), television viewing <2 hours/day (67% of participants), and walking ≥30 minutes/day (56% of participants). Sixty-one (7%) women reported none of the beneficial behaviors, 298 (33%) reported one, 368 (41%) reported two, and 175 (19%) reported all three. Compared with those who reported none, women reporting all three beneficial behaviors had a markedly reduced risk of retaining ≥5kg (OR 0.23, 95% CI = 0.08–0.66). Odds ratios decreased linearly across those reporting 0, 1, 2, or all 3 beneficial behaviors (p for trend <0.001, see Figure).
Figure 1.
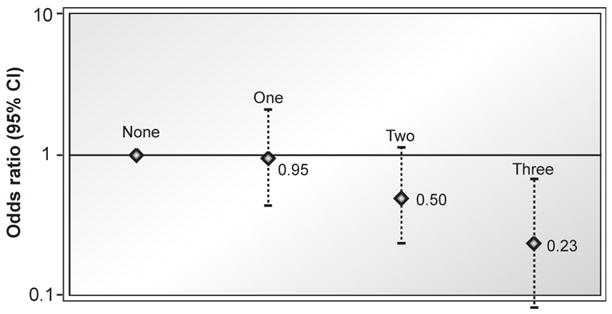
Odds ratios of retaigning at least 5 kg at 1 year postpartum according to the number of beneficial postpartum behaviors. Beneficial behaviors defined as (1) being below the median for trans fat intake, (2) watching less than 2 hours of television daily, and (3) walking at least 30 minutes daily (p for trend across categories <0.001).
DISCUSSION
In this prospective study, television viewing and trans fat intake in the early postpartum period were directly associated, and walking inversely associated, with substantial weight retention at 1 year postpartum. Effects of these three behaviors were additive, as women who watched less than 2 hours of television, walked at least 30 minutes, and consumed below the median amount of trans fat daily had an estimated 77% reduced odds of retaining at least 5kg compared with women who reported none of the beneficial behaviors.
Postpartum weight retention may be more physiologically harmful than weight gained at other times in life. Excess weight retained after pregnancy appears to be preferentially deposited centrally,3,4 and central adiposity is tightly linked to insulin resistance and increased cardiovascular disease risk.27,28 These associations highlight the importance of identifying modifiable risk factors for postpartum weight retention.
Inactivity, primarily television viewing, and physical activity are important and independent determinants of body weight, weight gain, and risk of obesity-related diseases such as type 2 diabetes mellitus.12,13,29 However, few studies of postpartum weight retention have considered physical activity,7,9 and only one included a measure of inactivity.10 Small intervention studies among overweight postpartum women have shown that exercise is safe and promotes weight loss, but the extent to which new mothers are able to maintain rigorous exercise programs is unclear.1,30 In the postpartum period, many women can include walking in their daily routine (e.g., pushing the child in a stroller) and walking is efficacious in reducing disease risks.31–33 In contrast to walking, light/moderate and vigorous activity decreased from pre-pregnancy to postpartum in this cohort, and were not associated with decreased risk of substantial weight retention. It is not clear why more vigorous activities did not also protect against weight retention, as physical activity has been shown to promote postpartum weight loss in other studies.30 It is possible that women who were having more difficulty losing weight either undertook or reported more light/moderate and vigorous activities.
To our knowledge, no reports exist on associations of television viewing with postpartum weight retention. Previous studies among children and nonpregnant adults have related increased television viewing to overweight and obesity.34–38 Moreover, randomized controlled trials among children have shown that television viewing is modifiable and that reduction in television viewing leads to a reduction in overweight.39,40
The few prior studies of diet and weight retention in the postpartum period have focused on diet quantity or energy intake,8–10 but have not investigated individual foods and nutrients, including fats, fiber and glycemic index. Diet quality may be easier to modify than energy intake,11,41 particularly among breastfeeding mothers as concerns remain that energy restriction may adversely influence milk production and infant growth.42 In the present study, higher trans fat intake was associated with increased risk of substantial weight retention. While evidence linking intake of trans fat with adverse blood cholesterol profiles and risk of coronary heart disease is well known, 43,44 recent studies suggest that trans fat intake also is linked with weight, weight gain, and increasing waist circumference in nonpregnant adults,15,16 perhaps by means of increasing systemic inflammation.45–47 Women may now be more informed about their trans fat intake, since as of January 1, 2006, the U.S. Food and Drug Administration requires that all food labels must list the content of trans fat.
The relatively large sample size, prospective data collection, and inclusion of multiple potential confounding variables are strengths of this study. By examining television viewing, walking, and diet simultaneously, this study has demonstrated that each behavior yields incremental benefit. However, although 21% of mothers were nonwhite, their educational and income levels were relatively high, thus results may not be generalizable to socioeconomically disadvantaged populations. In this population, women had somewhat lower weight retention than has been reported elsewhere. Just over half of the eligible study population provided enough information to allow their inclusion in this analysis. Included participants did not substantially differ from the overall population in measured characteristics, but may have differed in their weight retention. Both pre-pregnancy and post partum weight were by self-report, but any underreporting bias would probably have been similar for both pre-pregnancy and postpartum weight. The physical activity questions used here have not been validated in other pregnant populations. Although we analyzed walking according to average daily activity, women reported their physical activity in hours per week; it is not possible to determine whether the most important exposure is by daily or cumulative weekly walking. Associations of television viewing with weight retention may differ among women viewing more television, as is common in other populations. Trans fat intake may be a marker for other unhealthy dietary or other lifestyle behaviors rather than causally associated with weight gain. Because PrimeScreen is a brief screening tool, it may not assess intake of total fat as well as for individual types of fat. Further evaluation of associations of postpartum weight retention with other nutrients such as fiber and total fat is warranted in future studies.
Improved understanding of common and modifiable determinants of weight gain is critical to the design of interventions to prevent obesity. The puerperal period is a time when women are particularly receptive to behavior change recommendations,48 and thus may provide an excellent opportunity to improve weight-related behaviors. Future interventions should test the potential benefits of limited television viewing, reduced trans fat intake, and frequent walking to reduce the risk of substantial weight retention and promote long-term maternal health.
Acknowledgments
We thank the participants and staff of Project Viva as well as Sheryl Rifas-Shiman for programming support. We appreciate the helpful suggestions regarding study design and manuscript revisions from Dr. Erica Gunderson, Division of Research, Kaiser Permanente of Northern California; and Dr. Walter Willett, Department of Nutrition, Harvard School of Public Health.
This study was supported by grants from the U.S. National Institutes of Health (HD 34568, HL 64925, HL 68041, HD 44807), the Robert Wood Johnson Foundation, Harvard Medical School (the Division of Nutrition and the Robert H. Ebert Fellowship), and the Harvard Pilgrim Health Care Foundation.
No financial conflict of interest was reported by the authors of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Ann Behav Med. 2003;26(2):149–59. doi: 10.1207/S15324796ABM2602_07. [DOI] [PubMed] [Google Scholar]
- 2.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev. 2000;22(2):261–74. doi: 10.1093/oxfordjournals.epirev.a018038. [DOI] [PubMed] [Google Scholar]
- 3.Gunderson EP, Murtaugh MA, Lewis CE, Quesenberry CP, West DS, Sidney S. Excess gains in weight and waist circumference associated with childbearing: The Coronary Artery Risk Development in Young Adults Study (CARDIA) Int J Obes Relat Metab Disord. 2004;28(4):525–35. doi: 10.1038/sj.ijo.0802551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. Jama. 1994;271(22):1747–51. [PubMed] [Google Scholar]
- 5.Linne Y, Barkeling B, Rossner S. Long-term weight development after pregnancy. Obes Rev. 2002;3(2):75–83. doi: 10.1046/j.1467-789x.2002.00061.x. [DOI] [PubMed] [Google Scholar]
- 6.Ohlin A, Rossner S. Maternal body weight development after pregnancy. Int J Obes. 1990;14(2):159–73. [PubMed] [Google Scholar]
- 7.Schauberger CW, Rooney BL, Brimer LM. Factors that influence weight loss in the puerperium. Obstet Gynecol. 1992;79(3):424–9. doi: 10.1097/00006250-199203000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Olson CM, Strawderman MS, Hinton PS, Pearson TA. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. Int J Obes Relat Metab Disord. 2003;27(1):117–27. doi: 10.1038/sj.ijo.0802156. [DOI] [PubMed] [Google Scholar]
- 9.Boardley DJ, Sargent RG, Coker AL, Hussey JR, Sharpe PA. The relationship between diet, activity, and other factors, and postpartum weight change by race. Obstet Gynecol. 1995;86(5):834–8. doi: 10.1016/0029-7844(95)00283-W. [DOI] [PubMed] [Google Scholar]
- 10.Ohlin ARS. Trends in eating patterns, physical activity and socio-demographic factors in relation to postpartum body weight and development. Br J Nutr. 1994;71(4):457–470. doi: 10.1079/bjn19940155. [DOI] [PubMed] [Google Scholar]
- 11.Willett W. Nutritional Epidemiology. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 12.Littman AJ, Kristal AR, White E. Effects of physical activity intensity, frequency, and activity type on 10-y weight change in middle-aged men and women. Int J Obes Relat Metab Disord. 2005;29(5):524–33. doi: 10.1038/sj.ijo.0802886. [DOI] [PubMed] [Google Scholar]
- 13.Artal R, O'Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37(1):6–12. doi: 10.1136/bjsm.37.1.6. discussion 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr. 2003;78(5):920–7. doi: 10.1093/ajcn/78.5.920. [DOI] [PubMed] [Google Scholar]
- 15.Koh-Banerjee P, Chu NF, Spiegelman D, et al. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 U.S. men. Am J Clin Nutr. 2003;78(4):719–27. doi: 10.1093/ajcn/78.4.719. [DOI] [PubMed] [Google Scholar]
- 16.Memisoglu A, Hu FB, Hankinson SE, et al. Interaction between a peroxisome proliferator-activated receptor gamma gene polymorphism and dietary fat intake in relation to body mass. Hum Mol Genet. 2003;12(22):2923–9. doi: 10.1093/hmg/ddg318. [DOI] [PubMed] [Google Scholar]
- 17.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144(2):240–5. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 18.Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: results from a U.S. pregnancy cohort. Am J Epidemiol. 2004;160(8):774–83. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Medical Association. World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277(11):925–6. [PubMed] [Google Scholar]
- 20.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 21.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108(3):161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 22.Pereira MA, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Peterson KE, Gillman MW. Predictors of change in physical activity during and after pregnancy: Project Viva. Am J Prev Med. 2006 doi: 10.1016/j.amepre.2006.12.017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rifas-Shiman SL, Willett WC, Lobb R, Kotch J, Dart C, Gillman MW. PrimeScreen, a brief dietary screening tool: reproducibility and comparability with both a longer food frequency questionnaire and biomarkers. Public Health Nutr. 2001;4(2):249–54. doi: 10.1079/phn200061. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine. Nutrition during pregnancy. Washington, DC: National Academy Press; 1990. [Google Scholar]
- 26.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 27.Gunderson EP, Lewis CE, Murtaugh MA, Quesenberry CP, Smith West D, Sidney S. Long-term plasma lipid changes associated with a first birth: the Coronary Artery Risk Development in Young Adults study. Am J Epidemiol. 2004;159(11):1028–39. doi: 10.1093/aje/kwh146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Department of Health and Human Services. Physical activity and health: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services; 1996. [Google Scholar]
- 30.Larson-Meyer DE. Effect of postpartum exercise on mothers and their offspring: a review of the literature. Obes Res. 2002;10(8):841–53. doi: 10.1038/oby.2002.114. [DOI] [PubMed] [Google Scholar]
- 31.Pereira MA, Kriska AM, Day RD, Cauley JA, LaPorte RE, Kuller LH. A randomized walking trial in postmenopausal women: effects on physical activity and health 10 years later. Arch Intern Med. 1998;158(15):1695–701. doi: 10.1001/archinte.158.15.1695. [DOI] [PubMed] [Google Scholar]
- 32.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282(15):1433–9. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 33.Swartz AM, Strath SJ, Bassett DR, et al. Increasing daily walking improves glucose tolerance in overweight women. Prev Med. 2003;37(4):356–62. doi: 10.1016/s0091-7435(03)00144-0. [DOI] [PubMed] [Google Scholar]
- 34.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–91. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 35.Fung TT, Hu FB, Yu J, et al. Leisure-time physical activity, television watching, and plasma biomarkers of obesity and cardiovascular disease risk. Am J Epidemiol. 2000;152(12):1171–8. doi: 10.1093/aje/152.12.1171. [DOI] [PubMed] [Google Scholar]
- 36.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161(12):1542–8. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 37.Crespo CJ, Smit E, Troiano RP, Bartlett SJ, Macera CA, Andersen RE. Television watching, energy intake, and obesity in U.S. children: results from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2001;155(3):360–5. doi: 10.1001/archpedi.155.3.360. [DOI] [PubMed] [Google Scholar]
- 38.Gortmaker SL, Must A, Sobol AM, Peterson K, Colditz GA, Dietz WH. Television viewing as a cause of increasing obesity among children in the United States, 1986–1990. Arch Pediatr Adolesc Med. 1996;150(4):356–62. doi: 10.1001/archpedi.1996.02170290022003. [DOI] [PubMed] [Google Scholar]
- 39.Gortmaker SL, Peterson K, Wiecha J, et al. Reducing obesity via a school-based interdisciplinary intervention among youth: Planet Health. Arch Pediatr Adolesc Med. 1999;153(4):409–18. doi: 10.1001/archpedi.153.4.409. [DOI] [PubMed] [Google Scholar]
- 40.Robinson TN. Reducing children's television viewing to prevent obesity: a randomized controlled trial. Jama. 1999;282(16):1561–7. doi: 10.1001/jama.282.16.1561. [DOI] [PubMed] [Google Scholar]
- 41.Beresford SA, Curry SJ, Kristal AR, Lazovich D, Feng Z, Wagner EH. A dietary intervention in primary care practice: the Eating Patterns Study. Am J Public Health. 1997;87(4):610–6. doi: 10.2105/ajph.87.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butte NF. Dieting and exercise in overweight, lactating women. N Engl J Med. 2000;342(7):502–3. doi: 10.1056/NEJM200002173420709. [DOI] [PubMed] [Google Scholar]
- 43.Willett WC, Stampfer MJ, Manson JE, et al. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet. 1993;341(8845):581–5. doi: 10.1016/0140-6736(93)90350-p. [DOI] [PubMed] [Google Scholar]
- 44.Mensink RP, Katan MB. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N Engl J Med. 1990;323(7):439–45. doi: 10.1056/NEJM199008163230703. [DOI] [PubMed] [Google Scholar]
- 45.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79(6):969–73. doi: 10.1093/ajcn/79.6.969. [DOI] [PubMed] [Google Scholar]
- 46.Mozaffarian D, Pischon T, Hankinson SE, et al. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr. 2004;79(4):606–12. doi: 10.1093/ajcn/79.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engstrom G, Hedblad B, Stavenow L, Lind P, Janzon L, Lindgarde F. Inflammation-sensitive plasma proteins are associated with future weight gain. Diabetes. 2003;52(8):2097–101. doi: 10.2337/diabetes.52.8.2097. [DOI] [PubMed] [Google Scholar]
- 48.Lumley J, Oliver SS, Chamberlain C, Oakley L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2004;(4):CD001055. doi: 10.1002/14651858.CD001055.pub2. [DOI] [PubMed] [Google Scholar]


