Abstract
Embryonic stem (ES) cells have been investigated in many animal models of injury and disease. However, few studies have examined the ability of pre-differentiated ES cells to improve functional outcome following traumatic brain injury (TBI). The purpose of the present study was to compare the effect of murine ES cells that were pre-differentiated into GABAergic neurons or astrocytes on functional recovery following TBI. Neural and astrocyte induction was achieved by co-culturing ES cells on a bone marrow stromal fibroblast (M2-10B4) feeder layer and incubating them with various mitogenic factors. Rats were initially prepared with a unilateral controlled cortical contusion injury of the sensorimotor cortex or sham procedure. Rats were transplanted 7 days following injury with ∼100K GABAergic neurons, astrocytes, fibroblasts, or media. Animals were assessed on a battery of sensorimotor tasks following transplantation. The stromal fibroblast cells (M2-10B4), as a control cell line, did not differ significantly from media infusions. Transplantation of GABAergic neurons facilitated complete and total recovery on the vibrissae-forelimb placing test as opposed to all other groups, which failed to show any recovery. It was also found that GABAergic neurons reduced the magnitude of the initial impairment on the limb use test. Histological analysis revealed infiltration of host brain with transplanted neurons and astrocytes. The results of the present study suggest that transplantation of pre-differentiated GABAergic neurons significantly induces recovery of sensorimotor function; whereas, astrocytes do not.
Keywords: GABA, Traumatic Brain Injury, Recovery of Function, Embryonic Stem Cells, Rat, Astrocytes
INTRODUCTION
Stem cell transplants have been evaluated using many different methods in traumatic brain injury (TBI) models. A thorough review on the therapeutic use of stem cell transplants in TBI has recently been published [18]. Previous studies using undifferentiated stem cells have shown that cells usually turn into glial cells in the brain following TBI [6]. However, recent studies have investigated pre-differentiated cell lines. The murine neural stem cell (NSC) clone C17.2 was examined in non-immunosuppressed mice using a lateral controlled cortical impact (CCI) model [24]. Transplantation of the NSCs or control cell line occurred 3 days following injury. This study showed that NSCs survived in the lesion cavity for up to 13 weeks following transplantation and improved motor but not cognitive function. Investigating the capabilities of neuronal and glial precursors in an ES cell transplantation study, we reported that neuronal and glial precursors significantly improved sensorimotor function but not cognitive function following CCI of the sensorimotor cortex (SMC) [13]. Specifically, a mixed culture of neuronal and glial precursors facilitated an initial reduction in the magnitude of behavioral impairments in the bilateral tactile removal and locomotor placing tests, and significantly improved functional improvement on the vibrissae-forelimb placing test. The results of this study were similar to other cell transplantation studies using NSCs [20,24] which showed improvements in motor but not cognitive functions. However, a recent study that engineered neural progenitor cells to express glial-derived neurotrophic factor (GDNF) has been shown to increase the survival of the transplanted cells and to improve cognitive performance but not motor function [2].
Pre-differentiated ES cells have been shown to facilitate motor recovery following TBI [13,24]. However, it is unknown if the observed functional improvement is produced by neuronal or glial cell lineages in the mixed culture. This can be accomplished by further differentiating the mixed culture into specialized phenotypes. It has been shown that embryoid bodies, which form in culture can be differentiated and separated in culture by using a differentiation protocol that exposes the cells to various mitogens (basic fibroblast growth factor (bFGF), sonic hedgehog (SHH), FGF8, neurotrophin-4 (NT4) and brain derived neurotrophic factor (BDNF)) [4]. Astrocytes can be grown with the addition of bFGF, epidermal growth factor (EGF), and ciliary neurotrophic factor (CNTF) [4].
Transplantation of astrocytes may be beneficial because they are a major source of nerve growth factor following TBI [9]. It has also been shown that GDNF is neuroprotective and is released by astrocytes [15]. In addition to being neuroprotective, GDNF expressing cells are able to engraft into a lesioned striatum, give rise to neuronal cell lineages, prevent the loss of dopaminergic neurons, and reduces behavioral impairments in a mouse model of Parkinson’s disease [1]. It has also been suggested that transplanted undifferentiated glial restricted cells, which became oligodendrocytes and astrocytes, have the ability to survive in the lesion cavity, reduce astrocytic scarring, and reduce the expression of inhibitory proteoglycans [11].
One potential neuronal phenotype that may be beneficial when transplanted following TBI is gamma-aminobutyric acid (GABA). The rationale for transplanting GABAergic neurons is that they are the major inhibitory neurotransmitter in the brain. GABAergic neurons are found throughout the CNS and occur in high concentrations in the cortex [21]. In addition, GABAergic neurons have also be shown to be anticonvulsant by antagonizing glutamate and GABAA receptors [27], and are capable of surviving certain types of injuries (e.g. focal ischemic injury) [8]. A recent study has shown that GABAergic neurons can be grown in culture and can be transplanted and survive in the cortex of the mature rodent brain [21]. Three days following transplantation, the GABAergic transplants survived and produced spines, though there were no behavioral analyses performed to determine the impact of these transplants on behavioral functioning. Thus, the purpose of the present study was to examine the ability of differentiated GABAergic neurons or astrocytes to improve recovery of function when transplanted following TBI. Bone marrow derived stromal fibroblasts (M2-10B4) were growth arrested and used as a cell transplant control in one group of injured animals.
MATERIALS & METHODS
Cell Culture and Differentiation
Murine ES cells (D3, ATCC) were transfected in the presence of FuGENE 6 (Roche) to express green fluorescent protein (GFP) [10,13,22,23]. Transfected colonies were selected as described previously [13] (see Fig. 1C). Induction of neuronal lineage was achieved by culturing the ES cells as a single cell suspension at a density of 50 cells/cm2 in 15% serum replacement medium (SRM) on a stromal feeder layer (M2-10B4, murine, growth arrested with mitomycin) for 5 to 6 days depending on its targeted differentiation (GABAergic neurons or astrocytes). Further differentiation of the cells was achieved by using standard methods [4]. To culture GABAergic neurons, ES cells were grown on a stromal feeding layer for 5 days in SRM (see Fig. 1A-B). The ES cells were then transferred to neuralbasal medium + N2 supplement (Gibco) that was further supplemented with 10 ng/mL basic fibroblast growth factor (bFGF) for 4 days. Afterwards, 200 ng/ml sonic hedgehog (SHH) and 100 ng/ml FGF8 were added for 2 more days. Differentiation to GABAergic neurons was induced by withdrawing mitogen and adding 20 ng/ml neurotrophin-4 (NT4) and 20 ng/ml brain derived neurotrophic factor (BDNF) (see Fig. 1D-E).
Fig. 1.

Histological plates. (A) Differentiating neural stem cells, first day after re-plating on polyornithin/fibronectin. Scale bar is 100 μm. (B) GABAergic progenitors, expressing GAD67 following mitogen treatment. Scale bar is 50 μm. (C) Embryoid body, 11 days after plating expressing MAP2. Scale bar is 50 μm. (D) Cells expressing GAD67 after differentiation with Shh. Scale bar is 50 μm. (E) Astrocyte progenitors, expressing GFAP. Scale bar is 50 μm. (F) Dorsal view of the brain showing unilateral contusion site (black circle) and both transplantation sites (red asterisks).
Astrocytes were obtained by culturing the ES cells on the stromal feeding layer for 6 days in SRM. ES cells were then transferred into a N2 medium which was supplemented with 10 ng/mL bFGF and 20 ng/mL epidermal growth factor (EGF) for 3 days. ES cells were then cultured in N2 medium with bFGF, EGF, and 20 ng/mL ciliary neurotrophic factor (CNTF) for an additional 3 days. Afterwards, all the growth factors with the exception of CNTF were removed.
Growth arrested stromal cells (bone marrow stromal fibroblasts, M2-10B4), for transplantation, were obtained by growing cells to 100% confluency (contact inhibition - induced growth arrest)[4]. These cells were identical to the feeder layer that the neurons and astrocytes were grown on and were used as a cellular control to determine if transplantation of a general cell line would produce improvements in recovery of function.
All cells were allowed to proliferate in culture for up to 1 week. Cells were then collected and transplantation concentrations were adjusted to 20,000 cells/μL by using a hemocytometer. Cells not transplanted were replated and cultured for analysis.
Subjects
Thirty-three male Sprague-Dawley rats, approximately 3 months old, (mean weight = 358.94 g, SEM = 6.9 g) were used in this experiment. The animals were maintained on a standard 12 hr light/dark cycle with food and water available ad lib. TBI, transplantation, and behavioral tests were conducted during the light-on cycle. All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee and was conducted in a facility certified by the American Association for the Accreditation of Laboratory Animal Care.
Surgery
A standard CCI model was used in this experiment that produced a moderate injury [13]. Subjects were anesthetized with a ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) cocktail. Once the animal was unresponsive (no ocular or pedal reflexes) the head was shaved and scrubbed with 70% alcohol and Betadine. Animals were then placed into a stereotaxic device and a midline incision was made in the skin and underlying fascia. A 6.0 mm circular craniotomy was made unilaterally, centered 3.0 mm directly lateral to bregma over the left SMC, using a Dremel motor tool and a specially designed drill bit designed to prevent damage to the meninges/cortex (see Fig. 1F). A sterile 3.0 mm in diameter, stainless steel, impactor tip was attached to a piston activated with compressed air (2.5 m/s) and positioned on the surface of the intact dura. The piston was activated causing the impactor tip to make contact and compress the cortex for approximately 0.5 sec, resulting in a 2.0 mm compression of the SMC. If necessary, any bleeding was controlled using sterile gauze soaked in cold saline. The incision was temporarily closed using surgical staples and treated with triple antibiotic ointment. Animals were then placed on isothermic (Braintree Scientific Inc.) heating units (37°C) in order to maintain body temperature during post-surgical recovery. Sham animals were anesthetized, prepared for surgery, placed into the stereotaxic device, given a midline incision, stapled closed and allowed to recover on the heating pad.
Transplantation
GABAergic neurons, astrocytes, stromal cells, or media were transplanted 7 days following injury [13]. Animals were anesthetized with ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and prepared for surgery. The injured animals were randomly assigned to receive a transplantation of GABAergic neurons, astrocytes, stromal cells, or media. Once the animal was unresponsive (no ocular or pedal reflexes), the surgical staples were removed, a midline incision was made, and the skin and fascia were retracted. A 10 μL Hamilton syringe and a microdrive (Stoelting Instruments) was attached to the stereotaxic device and used to perform all transplantations. The syringe was carefully loaded with GABAergic neurons (20K/μl), astrocytes (20K/μL), stromal cells (20K/μL), or media. The tip of the syringe was centered +3.0 mm lateral to bregma over the center of the craniotomy. Approximately 100K cells (GABAergic, astrocytes, or stromal) (2.5 μL) or 2.5 μL of media was deposited at 2 two sites (+1.0 mm anterior to bregma and -1.0 mm posterior to bregma) in the injured cortex at a depth of -3.0 mm below the skull surface (see Fig. 1F). The deposits were made at a rate of 0.5 μL/min. The syringe was left in place for 2 min following the deposit before being slowly raised from the site. Animals were sutured using nylon suture material and allowed to recover on a heating unit. Sham animals were once again anesthetized, placed into the stereotaxic device, given a midline incision, sutured, and allowed to recover on a heating unit. No immunosuppression was administered post-surgery. Following transplantation, the following groups had been created: TBI + GABAergic neurons (n = 8), TBI + astrocytes (n =8), TBI + stromal cells (n = 5), TBI + media (n = 7), and sham (n = 5). All behavioral testing and histological analysis was done without any knowledge of group assignments.
Vibrissae-Forelimb Placing Test
This test measures sensorimotor integration and has also been described in great detail [5,12,13,25]. Animals were tested on post-transplant days 2, 4, 6, 10, 14, 21, 28, and 35. Subjects were held by the trunk allowing free movement of the forelimbs. The chin was supported and the head elevated 45°upwards to eliminate unwanted facial stimulation. Each forelimb was independently tested for placing reactions by orienting one side of the animal toward a Plexiglas surface and slowly moving the animal up and down causing the vibrissae to touch the surface. Intact animals consistently produced a placing response with the ipsilateral forelimb to the side of stimulation upon vibrissae contact. The placing reaction involves the animal lifting the forelimb and placing it on the Plexiglas surface. If no placing reaction occurred within a 5 sec trial period, an unsuccessful trial was recorded.
Limb use Asymmetry Test
This task measures somatosensory dysfunction following injury and is a sensitive measure of limb use asymmetry following unilateral brain injury and stroke [16,17,25]. Animals were tested on post-transplantation days 5 and 12. For this task, an animal was placed in a square glass observation chamber (25 cm × 25 cm × 25 cm) and allowed to explore for 5 min while being videotaped. During exploration of the chamber animals will rear, pressing one or both forepaws against the vertical surface [25]. Intact animals will use their forelimbs (right vs. left) equally, and do not show a limb use asymmetry. During video analysis, the number of times each forelimb was placed on the vertical surface was recorded. The data are represented as the percentage of preference for the contralateral limb, calculated using the following formula: [contralateral forelimb/(contralateral + ipsilateral)] × 100. A derived score of 50% reflects equal use of both forelimbs, a score greater than 50% represents greater preference for the limb contralateral to the site of injury, while a score less than 50% indicates greater use of the limb ipsilateral to the injury.
Histology
At 42 days post-transplantation, animals were anesthetized with Nembutal (100 mg/kg, i.p.) and transcardially perfused with 0.9% phosphate buffered saline followed by 10% phosphate buffered formalin. Brains were removed from the cranium and post-fixed in formalin for 4 hrs and then cryopreserved in 30% sucrose for 3 days prior to sectioning.
Immunocytochemistry, Immunohistochemistry, and Immunofluorescence
The following antibodies were used for immunostaining procedures. Anti-MAP-2 rabbit polyclonal antibodies (immunoreactivity with neuronal MAP-2A and MAP-2B) (dilution 1: 2000) (Chemicon), mouse monoclonal antibodies against mouse surface antigen M2 (1:100) (Developmental Studies Hybridoma Bank), anti-glutamate decarboxylase (GAD67) rabbit polyclonal antibodies 1:1000 (Chemicon), anti-GFAP, rabbit polyclonal antibodies 1:1000 (Chemicon). For immunofluorescence all primary antibodies were applied in 1:100 dilutions.
Brains were cut with a cryostat into 30 micron coronal slices and were then processed as free-floating sections [13,22]. Immunostaining was performed using the Elite ABC Kit (Vector Laboratories, Burlingame, CA). Initially, sections were washed for 10 min two times in phosphate buffered saline with 0.3% Triton X-100 (PBST) and incubated for 30 min in ice cold 100% methanol containing 1% H202 to abolish endogenous peroxidase activity. Sections were then washed twice in PBST for 10 min and incubated for 1 hr with blocking solution, 10% normal goat serum in PBST. Sections were then incubated overnight at 4°C with primary antibodies. Sections were then washed three times for 15 min in PBST and incubated for an hour with secondary biotinylated antibodies. Sections were washed three more times for 15 min with PBST and incubated in ABC reagent (avidin-biotinylated horseradish peroxidase complex) for 2 hrs. After staining, tissue antigen was visualized with a DAB Substrate Kit for Peroxidase (Vector Laboratories). Sections were then air dried and mounted with Permount (Fisher Scientific).
Dual immunofluorescent staining techniques were applied to locate cells by expression of GAD-67 (GABAergic neurons), GFAP (astrocytes), and M2. Secondary antibodies conjugated with fluorescent dyes FITC (green fluorescence) and Texas Red (red fluorescence) (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were applied in 1:100 dilution for 1 hr at room temperature. Sections were rinsed with dH2O and mounted using anti-fading Gel/Mount (Biomeda, Foster City, CA). Images were captured using a Spot digital camera. For doublestained specimens and for subcellular resolution a Zeiss LSM 510 confocal laser-scanning microscope was used.
Statistical Analysis
Analysis of variance (ANOVA) tests were preformed using the procedures for general linear models (SPSS 14.0 for windows) with options for repeated measures where required. The between groups factor was group (GABAergic neurons, astrocytes, stromal cells, media, or sham) while the within groups factor was day of testing. Huyn-Feldt probabilities (HFP) were used for assessing repeated measures factors [13]. Post-hoc analyses were performed using Tukey′s test for comparison of means. A probability value of p < 0.05 was considered significant for all statistical tests. All data are shown as mean ±SEM.
RESULTS
Initial analyses indicated that there were no significant differences in behavioral performance between the animals that received stromal cells and media infusions. Comparison of limb use asymmetry with repeated measures ANOVA of the stromal (n = 4) and media groups (n = 6) revealed no significant differences for group [F(1,8) = 0.40, p > 0.55], day [F(1,8) = 0.16, p > 0.70], or the group × day interaction [F(1,8) = 0.02, p > 0.90]. The same was also true for the vibrissae-forelimb placing data. The effects for group [F(1,8) = 0.64, p > 0.45], day [F(1,8) = 0.64, p > 0.47], and the group × day interaction [F(1,8) = 0.64, p > 0.47] were all nonsignificant. Thus, the groups were combined to create a single injured-control group. Additionally, 4 animals were excluded from data analyses due to variability in the site of injury (1 injury was partially bilateral (astrocyte group), 2 injury cavities extended through the corpus callosum and into the dorsal striatum (1 in GABA group and 1 in media group), and one animal was removed during testing because of morbidity (stromal group). Thus, all subsequent analyses were conducted with the following groups: TBI + GABAergic neurons (n = 7), TBI + astrocytes (n = 7), TBI + stromal cells/media (n = 10), and sham (n = 5).
Vibrissae-Forelimb Placing Test
The percent of unsuccessful contralateral placing attempts was examined in a 4 × 8 ANOVA, including groups (TBI + GABAergic neurons, TBI + Astrocytes, TBI + media or stromal cells, shams) and the day of testing following transplantation (days 2, 4, 6, 10, 14, 21, 28, 35) as factors in the analysis, treating day as a repeated measure. Animals became more efficient at placing the contralateral forelimb on successive trials; the main effect for day was statistically significant [F(2.49, 62.25) = 27.24, p < 0.001]. Unilateral contusions produced significant impairments in the ability to place the contralateral forelimb; main effect of group was statistically significant [F(3,25) = 376.03, p < 0.0001]. There was also a significant difference in the rate of recovery; the group × day interaction was significant [F(6.12,62.25) = 21.46, p < 0.0001] (Fig. 2). Post-hoc comparisons revealed that transplanted GABAergic neurons facilitated a complete and total recovery of function compared to the astrocyte and injured control groups. Compared to the astrocyte and injured control groups, the recovery of the GABAergic group was statistically significant at days 28 and 35 (p < 0.001). Additionally, the performance of the GABAergic group was not statistically significant compared to the shams on the same days (28 and 35; p > 0.05). The astrocyte group did not show any significant improvements compared to the injured controls on all testing days (p > 0.05). Moreover, the astrocyte group and injured controls were significantly impaired compared to shams on all testing days 2 to 35 (p > 0.05). There were no impairments seen with the ipsilateral forelimb.
Fig. 2.
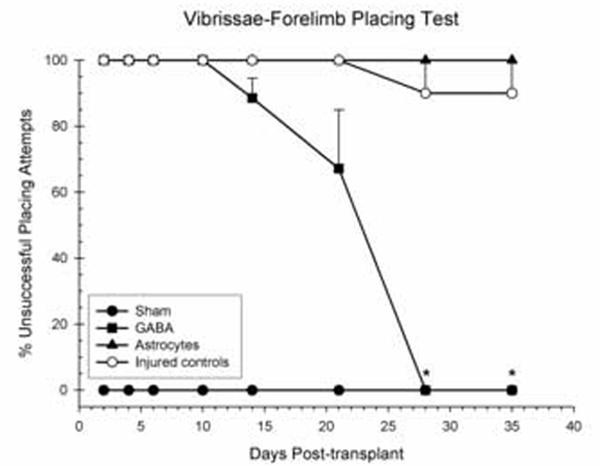
Shown is the effect of GABAergic neurons, astrocytes, or injured controls (stromal cells or media) transplanted 1 week following unilateral CCI of the SMC or sham surgery on the vibrissae-forelimb placing test. Plotted for each group is the mean (±SEM) percentage of unsuccessful placing attempts per test day. Injury significantly impaired performance on this test. Transplantation of GABAergic neurons induced a complete and total recovery by day 28 (*p < 0.05).
Limb-use Asymmetry Test
Limb-use asymmetries were analyzed using a 4 × 2 ANOVA including groups (TBI + GABAergic neurons, TBI + Astrocytes, TBI + media or stromal cells, sham) and day of testing following transplantation (day 5, day 12) as factors in the analysis, treating day following transplantation as the repeated measure. Unilateral contusions of the SMC produced significant deficits in the animals′ ability to place the contralateral forelimb on the glass when rearing; the main effect of group was statistically significant [F(3,25) = 9.40, p < 0.001] (Fig. 3). However, the effect of day [F(1,25) = 0.02, p > 0.90] and the group × day interaction was not significant [F(3,25) = 0.06, p > 0.98]. Post-hoc comparison of the significant main effect for group revealed that transplanted GABAergic neurons significantly reduced the asymmetry of the contralateral forelimb when compared to the control group (p = 0.001). Moreover, transplantation of GABAergic neurons following CCI resulted in forelimb asymmetries that were not significantly different from the sham group (p > 0.12). Transplantation of astrocytes did not significantly reduce forelimb asymmetries compared to injured controls (p > 0.06).
Fig. 3.

Shown is the effect of GABAergic neurons, astrocytes, or injured controls (stromal cells or media) transplanted 1 week following unilateral CCI of the SMC or sham surgery on the forelimb preference test (mean percentage ±SEM). The 50% point (represented by the line) indicates equal placing for either forelimb, no asymmetry in forelimb use. Preference for the contralateral forelimb is represented in the range above 50% (down arrow), ipsilateral forelimb preference is represented in the range below 50% (up arrow). Injury significantly impaired the use of the contralateral forelimb on this test, resulting in an increased ipsilateral bias. Transplantation of GABAergic neurons significantly reduced the ipsilateral bias compared to injured controls (* = p < 0.05). The GABA group was not significantly different from shams on either day (^).
Histological Analysis
Analysis of the brain tissue revealed numerous murine astrocytes and GABAergic neurons within their respected transplant groups. Fluorescent microscopy revealed that in the brains transplanted with astrocytes that there was extensive immunoflourescence for GFAP (Fig. 4A & D) and murine specific M2 (Fig. 4B & E) and when merged the images reveal substantial co-expression in the borders of the developing injury cavity (Fig. 4C & F). Similar results were observed in the GABA transplant group. GABAergic neurons can be seen expressing GAD67 at the transplant site (Fig 4G & J), expression of the M2 antigen is also present within the lesion cavity (Fig. 4H & K). When merged the images reveal transplanted GABAergic neurons co-expressing GAD67 and M2 (Fig. 4I & L).
Fig. 4.

Histological plates. (A, D) GFAP expression around the developing injury cavity (*). (B, E) Expression of murine specific antibody (M2) at the transplant site. (C, F) Merged image showing astrocytes expressing both M2 and GFAP in border of the lesion cavity. (G, J) Expression of GAD67, a marker for GABA immunoreactivity in the transplanted cortex (H, K) Expression of murine specific antibody (M2) at the transplant site. (I, L) Merged image showing neurons expressing both GABA and M2 immunofluorescence in the transplanted cortex. Scale bar for A-F is 100μm; scale bar for G-L is 50μm
DISCUSSION
The present study revealed that transplanted GABAergic neurons significantly improved recovery of sensorimotor function following unilateral CCI of the SMC, while transplantation of astrocytes did not. GABAergic neurons significantly reduced the initial behavioral impairments on the forelimb use asymmetry test. Moreover, GABAergic neurons facilitated a complete and total recovery on the vibrissae-forelimb placement test, while animals transplanted with astrocytes did not show any recovery in the duration of testing.
The current results support and extend our earlier findings that showed that transplantation of neuronal and glial precursors facilitated recovery of function in tests of sensorimotor function [13]. In that study, transplantation of a mixed culture of neuronal and glial precursors dramatically and significantly reduced the initial behavioral impairments on the tactile removal and locomotor placing tests just 2 days following transplantation. These transplants also induced recovery of function on the vibrissae-forelimb placing tests starting on day 14 and proceeded to improve recovery until 80% of the deficit was recovered, compared to the mediainfused controls which failed to recover and showed a 100% deficit 35 days after transplantation [13]. Given that the methodologies of our previous experiment [13] are identical to those in the present study it allows for comparison across studies. Thus, our initial study with a mixed culture of cells showed 80% recovery on the forelimb placing test; whereas, in the present study the GABA transplants resulted in total recovery. Although speculative, the transplantation of GABAergic neurons may have provided slightly greater recovery of function than the mixed culture of neural and glial precursors. Clearly, in the present study the recovery of sensorimotor function is facilitated by ES cells pre-differentiated into GABAergic neurons and not astrocytes.
Several ES cell studies have examined functional recovery following transplantation in TBI models. Transplanted minced fetal cortical grafts (E16) have been shown to significantly improve neuromotor and cognitive functions following TBI in rats [26]. Ipsilateral and contralateral transplantation of NSCs showed significantly improved motor functions [20,24]. Histological analysis revealed that transplanted NSC′s survived for 13 weeks following transplantation. At 13 weeks NSCs transplanted ipsilaterally showed neuronal (NeuN) and astrocytic (GFAP) markers whereas contralaterally transplanted NSC′s only expressed neuronal markers [24]. Studies pre-differentiating ES cells, using all-trans retinoic acid (RA) to induce differentiation into neuron-like cells in vitro, have shown cell survivability [7,13], significantly improved motor [7] and sensorimotor function [13]. These grafted cells have also been shown to contain synaptic vesicles, which suggests that the have functional capability [7]. A recent study has shown that pre-differentiated ES cells transplanted following excitotoxic SCI act in a neuroprotective manner and provide antinociceptive and therapeutic effects [10]. Thus the results of the present study are consistent with findings seen in other transplantation studies.
Although the goal of the present study was not to examine the possible mechanisms of action of the transplanted cells the behavioral data may provide some indirect insight into possible mechanisms. The most dramatic result seen in this study was the complete and total recovery on the vibrissae-forelimb placing test following transplantation of GABAergic neurons; when no recovery was seen in animals transplanted with astrocytes and in the control animals suggesting possible integration with the remaining cortical-subcortical circuitry involved in regulating this behavior. The transplanted GABAergic neurons in the present study appear to have developed a large pyramidal morphology, possibly supporting the idea of re-integration with host tissue. This result is similar to the one seen in a study transplanting neuronal and glial precursors [13]; however, it is also similar to the pattern of recovery seen in a study administering MgCl2 following TBI, which also began on day 14 [12]. Although speculative, there is support for both re-establishment of circuitry and possible trophic mechanisms involving GABAergic neurons in the literature. A recent study has shown that GABAergic neurons transplanted into the rodent cortex started to develop spine formations at 3 days following transplantation [21]; thus, it is possible that beneficial behavioral effects seen in the present study were produced by integration. Injection of the GABA(A) receptor agonist, muscimol (0.1 μg/0.1 μL), in rats with a unilateral nigrostriatal lesion showed a decreased ipsilateral bias after the lesion on the limb use asymmetry test [19]. Another study showed that long lasting exposures to brain-derived neurotrophic factor (BDNF) accelerated the functional maturation of GABAergic transmission in embryonic hippocampal neurons through enhanced overlapping and better colocalization of N- and P/Q-type channels to vesicle release sites [3]. It is possible that GABAergic transmission may also be enhanced due to neurotrophic factors released following injury. A recent study transplanting rat marrow stromal cells (rMSC′s) has examined gene expression of BDNF and nerve growth factor (NGF) following TBI [14]. It was found that rMSC′s transplanted via cistern magnum enhanced local expression of BDNF and NGF [14]. Perhaps transplantation of different stem cell lines may facilitate the expression of BDNF and NGF which in turn may enhance GABAergic transmission. While the significant effects were seen early in the present study (on day 5 in the forelimb asymmetry test) further studies will be needed to identify exact mechanisms by which transplanted GABAergic neurons produced the significant recovery of function seen in the present study.
The transplantation of astrocytes in the present study failed to provide significant improvements in recovery of function following TBI. In fact, the transplanted astrocyte group was never significantly different than the injured controls on any of the behaviors examined. However, transplanted astrocytes were observed contributing to the glial scar that was forming at the boundary of the developing injury cavity. Thus, it can be assumed that the presence of these cells, or any growth factors, that they may have contributed to the injured brain had no affect on behavioral recovery. However, this does not preclude that transplantation of a larger number of astrocytes might improve recovery. Similarly, we also transplanted stromal cells as a cellular control, to control for any possible cell delivered factors or signals, which might contribute positively to the recovery process. Our results show that this was not the case, transplantation of stromal cells were not significantly different compared to the media-transplanted control group.
The results of the present study have shown that transplantation of a population of GABAergic neurons produces significant improvements in sensorimotor recovery following TBI. It was also found that transplanted GABAergic neurons and astrocytes survived in the traumatically injured brain. Additionally, transplantation of stromal cells (fibroblasts) as a control cell line resulted in no significant improvement in behavior over that provided by media infused into the other control group. This suggests that the beneficial effects observed in the present study were not caused by just cellular transplantation factors. The present study supports the growing body of research suggesting that stem cells, especially those driven to neuronal lineages, serve as an effective treatment for TBI through mechanisms that may not clearly involve functional integration.
ACKNOWLEDGEMENTS
We would like to thank Dena Strickland and Stacy Harrison for their help with the behavioral studies and Dr. Arlene Tan for her assistance on drafts of this manuscript. This research was supported by an AREA grant (R15) from NINDS to MRH (NS045647-01) and a grant from the North Carolina Biotechnology Center (2004-MRG-1104) to AKM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Akerud P, Canals JM, Snyder EY, Arenas E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson′s disease. J Neurosci. 2001;21:8108–8118. doi: 10.1523/JNEUROSCI.21-20-08108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bakshi A, Shimizu S, Keck CA, Cho S, LeBold DG, Morales D, Arenas E, Snyder EY, Watson DJ, McIntosh TK. Neural progenitor cells engineered to secrete GDNF show enhanced survival, neuronal differentiation and improve cognitive function following traumatic brain injury. Eur J Neurosci. 2006;23:2119–2134. doi: 10.1111/j.1460-9568.2006.04743.x. [DOI] [PubMed] [Google Scholar]
- [3].Baldelli P, Hernandez-Guijo JM, Carabelli V, Carbone E. Brain-derived neurotrophic factor enhances GABA release probability and nonuniform distribution of N- and P/Q-type channels on release sites of hippocampal inhibitory synapses. J Neurosci. 2005;25:3358–3368. doi: 10.1523/JNEUROSCI.4227-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–1207. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- [5].Barth TM, Jones TA, Schallert T. Functional subdivisions of the rat somatic sensorimotor cortex. Behav Brain Res. 1990;39:73–95. doi: 10.1016/0166-4328(90)90122-u. [DOI] [PubMed] [Google Scholar]
- [6].Cao Q, Benton RL, Whittemore SR. Stem cell repair of central nervous system injury. J Neurosci Res. 2002;68:501–510. doi: 10.1002/jnr.10240. [DOI] [PubMed] [Google Scholar]
- [7].Chiba S, Ikeda R, Kurokawa MS, Yoshikawa H, Takeno M, Nagafuchi H, Tadokoro M, Sekino H, Hashimoto T, Suzuki N. Anatomical and functional recovery by embryonic stem cell-derived neural tissue of a mouse model of brain damage. J Neurol Sci. 2004;219:107–117. doi: 10.1016/j.jns.2004.01.006. [DOI] [PubMed] [Google Scholar]
- [8].Frahm C, Haupt C, Witte OW. GABA neurons survive focal ischemic injury. Neuroscience. 2004;127:341–346. doi: 10.1016/j.neuroscience.2004.05.027. [DOI] [PubMed] [Google Scholar]
- [9].Goss JR, O′Malley ME, Zou L, Styren SD, Kochanek PM, DeKosky ST. Astrocytes are the major source of nerve growth factor upregulation following traumatic brain injury in the rat. Exp Neurol. 1998;149:301–309. doi: 10.1006/exnr.1997.6712. [DOI] [PubMed] [Google Scholar]
- [10].Hendricks WA, Pak ES, Owensby JP, Menta KJ, Glazova M, Moretto J, Hollis S, Brewer KL, Murashov AK. Predifferentiated Embryonic Stem Cells Prevent Chronic Pain Behaviors and Restore Sensory Function Following Spinal Cord Injury in Mice. Mol Med. 2006;12:34–46. doi: 10.2119/2006-00014.Hendricks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hill CE, Proschel C, Noble M, Mayer-Proschel M, Gensel JC, Beattie MS, Bresnahan JC. Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: survival, differentiation, and effects on lesion environment and axonal regeneration. Exp Neurol. 2004;190:289–310. doi: 10.1016/j.expneurol.2004.05.043. [DOI] [PubMed] [Google Scholar]
- [12].Hoane MR, Barbay S, Barth TM. Large cortical lesions produce enduring forelimb placing deficits in un-treated rats and treatment with NMDA antagonists or anti-oxidant drugs induces behavioral recovery. Brain Res Bull. 2000;53:175–186. doi: 10.1016/s0361-9230(00)00327-0. [DOI] [PubMed] [Google Scholar]
- [13].Hoane MR, Becerra GD, Shank JE, Tatko L, Pak ES, Smith M, Murashov AK. Transplantation of neuronal and glial precursors dramatically improves sensorimotor function but not cognitive function in the traumatically injured brain. J Neurotrauma. 2004;21:163–174. doi: 10.1089/089771504322778622. [DOI] [PubMed] [Google Scholar]
- [14].Hu DZ, Zhou LF, Zhu J, Mao Y, Wu XH. Upregulated gene expression of local brain-derived neurotrophic factor and nerve growth factor after intracisternal administration of marrow stromal cells in rats with traumatic brain injury. Chin J Traumatol. 2005;8:23–26. [PubMed] [Google Scholar]
- [15].Kim BT, Rao VL, Sailor KA, Bowen KK, Dempsey RJ. Protective effects of glial cell line-derived neurotrophic factor on hippocampal neurons after traumatic brain injury in rats. J Neurosurg. 2001;95:674–679. doi: 10.3171/jns.2001.95.4.0674. [DOI] [PubMed] [Google Scholar]
- [16].Kozlowski DA, Hilliard S, Schallert T. Ethanol consumption following recovery from unilateral damage to the forelimb area of the sensorimotor cortex: reinstatement of deficits and prevention of dendritic pruning. Brain Res. 1997;763:159–166. doi: 10.1016/s0006-8993(97)00377-6. [DOI] [PubMed] [Google Scholar]
- [17].Kozlowski DA, James DC, Schallert T. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci. 1996;16:4776–4786. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Longhi L, Zanier ER, Royo N, Stocchetti N, McIntosh TK. Stem cell transplantation as a therapeutic strategy for traumatic brain injury. Transpl Immunol. 2005;15:143–148. doi: 10.1016/j.trim.2005.09.003. [DOI] [PubMed] [Google Scholar]
- [19].Mehta A, Chesselet MF. Effect of GABA(A) receptor stimulation in the subthalamic nucleus on motor deficits induced by nigrostriatal lesions in the rat. Exp Neurol. 2005;193:110–117. doi: 10.1016/j.expneurol.2005.01.001. [DOI] [PubMed] [Google Scholar]
- [20].Muir JK, Raghupathi R, Saatman KE, Wilson CA, Lee VM-Y, Trojanowski JQ, Phillips MF, McIntosh TK. Terminally differentiated human neurons survive and integrate following transplantation into the traumatically injured rat brain. J Neurotrauma. 1999;16:403–414. doi: 10.1089/neu.1999.16.403. [DOI] [PubMed] [Google Scholar]
- [21].Muramatsu D, Sato Y, Hishiyama S, Miyamoto Y, Hisatsune T. Transplantation of GABAergic neurons into adult mouse neocortex. Exp Neurol. 2005;194:1–11. doi: 10.1016/j.expneurol.2005.01.025. [DOI] [PubMed] [Google Scholar]
- [22].Murashov AK, Pak ES, Hendricks WA, Owensby JP, Sierpinski PL, Tatko LM, Fletcher PL. Directed differentiation of embryonic stem cells into dorsal interneurons. FASEB J. 2005;19:252–254. doi: 10.1096/fj.04-2251fje. [DOI] [PubMed] [Google Scholar]
- [23].Murashov AK, Pak ES, Katwa LC. Parallel development of cardiomyocytes and neurons in embryonic stem cell culture. Biochem Biophys Res Commun. 2005;332:653–656. doi: 10.1016/j.bbrc.2005.04.167. [DOI] [PubMed] [Google Scholar]
- [24].Riess P, Zhang C, Saatman KE, Laurer HL, Longhi LG, Raghupathi R, Lenzlinger PM, Lifshitz J, Boockvar J, Neugebauer E, Snyder EY, McIntosh TK. Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery. 2002;51:1043–1052. doi: 10.1097/00006123-200210000-00035. [DOI] [PubMed] [Google Scholar]
- [25].Schallert T, Woodlee MT. Orienting and Placing. In: Whishaw IQ, Kolb B, editors. The Behavior of the Laboratory Rat. Oxford University Press; New York: 2005. pp. 129–140. [Google Scholar]
- [26].Sinson GP, Voddi M, McIntosh TK. Combined fetal neural transplantation and nerve growth factor infusion: Effects on neurological outcome following fluid-percussion brain injury in the rat. J Neurosurg. 1996;84:655–662. doi: 10.3171/jns.1996.84.4.0655. [DOI] [PubMed] [Google Scholar]
- [27].Suzuki F, Heinrich C, Boehrer A, Mitsuya K, Kurokawa K, Matsuda M, Depaulis A. Glutamate receptor antagonists and benzodiazepine inhibit the progression of granule cell dispersion in a mouse model of mesial temporal lobe epilepsy. Epilepsia. 2005;46:193–202. doi: 10.1111/j.0013-9580.2005.35504.x. [DOI] [PubMed] [Google Scholar]


