Abstract
The discovery of small molecule inhibitors of cytotoxicity induced by amyloid-β (Aβ) oligomers, either applied extracellularly or accumulated intraneuronally, is an important goal of drug development for Alzheimer's disease (AD), but has been limited by the lack of efficient screening methods. Here we describe our approach using two cell-based methods. The first method takes advantage of the unique ability of extracellularly applied Aβ oligomers to rapidly induce the exocytosis of formazan formed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). We employed a short protocol to quantify this toxicity, and quickly identified two novel inhibitors, code-named CP2 and A5, from two compound libraries. A second independent screen of the same libraries using our previously published MC65 protection assay, which identifies inhibitors of toxicity related to intracellular Aβ oligomers, also selected the same two leads, suggesting that both assays select for the same anti-Aβ oligomer properties displayed by these compounds. We further demonstrated that A5 attenuated the progressive aggregation of existing Aβ oligomers, reduced the level of intracellular Aβ oligomers, and prevented the Aβ oligomer-induced death of primary cortical neurons, effects similar to those demonstrated by CP2. Our results suggest that, when combined, the two methods would generate fewer false results and give a high likelihood of identifying leads that show promises in ameliorating Aβ oligomer-induced toxicities within both intraneuronal and extracellular sites. Both assays are simple, suitable for rapid screening of a large number of medicinal libraries, and amenable for automation.
Keywords: Alzheimer' disease, amyloid-β, oligomer, toxicity, small molecule, aggregation
1. Introduction
It is widely believed that various neurotoxicities inflicted by Aβ play an essential role in the pathogenesis of AD (Hardy and Selkoe, 2002). Misfolding and aggregation of Aβ, the 42 residue Aβ42 in particular, appear to be largely responsible for toxicity. Aβaggregation is a multistep process involving transient or metastable intermediates, variously referred to as oligomers, protofibrils, amyloid pores, or Aβ-derived diffusible ligands; these may eventually lead to fibril formation (Caughey and Lansbury, 2003). Importantly, not all Aβ assemblies or conformations are equally toxic (Chromy et al., 2003; Liu and Schubert, 2006). Recent studies have shown that neurological dysfunction and neuronal toxicity are more prominently linked to particular forms of soluble Aβ oligomers (Podlisny et al., 1998; Walsh et al., 2002; Dahlgren et al., 2002; Chromy et al., 2003; Kayed et al., 2004; Lacor et al., 2004; Lesné et al., 2006). In addition, intraneuronal Aβ oligomers, identified by biochemical and immunohistochemical methods (Takahashi et al., 2004; Billings et al., 2005; Maezawa et al., 2006), appear to play an early pathological role in AD (Wirths et al., 2004). To minimize the degree of Aβ toxicity in the brain, small molecular weight compounds capable of inactivating or disaggregating existing neurotoxic oligomers of Aβ are particularly attractive therapeutic candidates (Blanchard et al., 2004; Yang et al., 2005; Walsh et al., 2005; Liu and Schubert, 2006). Beneficial compounds might act by blockage of toxic structures, conversion of toxic Aβ assemblies into non-toxic moieties, or dissociation of such aggregates into constituent monomers. Such toxicity-blocking small molecules are also useful tools for the study of the toxic conformations of Aβ assemblies, the nature of which is currently unknown.
Cell culture models of toxicity remain the most effective approaches for the testing of candidate compounds. Cell-free methods, usually designed for screening of compounds that dissolve Aβ aggregates (oligomers, protofibrils and fibrils) or inhibit Aβ aggregation, run the risk of identifying compounds that generate or stabilize neurotoxic Aβ aggregates; as such, such agents might do more harm than good (Liu and Schubert, 2006). It is also conceivable that a group of promising compounds would simply block the toxic Aβ conformation without changing the Aβ aggregation state. Such beneficial compounds will not be selected by cell-free methods. We therefore employ cell-based methods as the first line of screening, followed by cell-free methods for further characterization of compounds. Here we describe a combination of our previously published MC65 protection assay (Maezawa et al., 2006) and an assay modified from a sensitive MTT formazan exocytosis assay (MTT-FE) (Liu and Schubert, 2006) to efficiently select small molecule compounds that show promises in modifying the toxicity and/or structure of Aβ oligomers.
2. Results
2.1. The rapid MTT-FE assay detects cytotoxicity induced by Aβ42 oligomers
Aβ peptides, in particular Aβ42, are highly metastable at 37°C in the presence of physiological salts; multiple structures of Aβ form in a given solution over time (Stine et al., 2003). Due to this progressive heterogeneity, in prolonged cultures the demonstrated Aβ-related toxicity cannot be attributed to specific Aβ assemblies. Therefore we set out to design a protocol with which the cell toxicity could be detected after a relatively short exposure to Aβ oligomers, during which the structural heterogeneity of Aβ in culture is minimized. It was previously reported that Aβ aggregates, but not unaggregated Aβ, dramatically enhance MTT-FE with the formation of “needle-like” crystals at the cell surface (Liu and Schubert, 1997; Liu and Piasecki, 2001; Liu and Schubert, 2006). However, whether Aβ oligomers would induce a higher degree of MTT-FE than Aβ fibrils, reflecting their stronger toxicity, had not been determined. For this purpose, we first used Neuro-2a (N2a) cells because their viability is more significantly reduced by oligomers than by fibrillar or unaggregated Aβ42 species (Dahlgren et al., 2002). Later we also performed experiments using MC65 and B12 neuroblastoma lines as well as mouse primary cortical neurons, which yielded similar results.
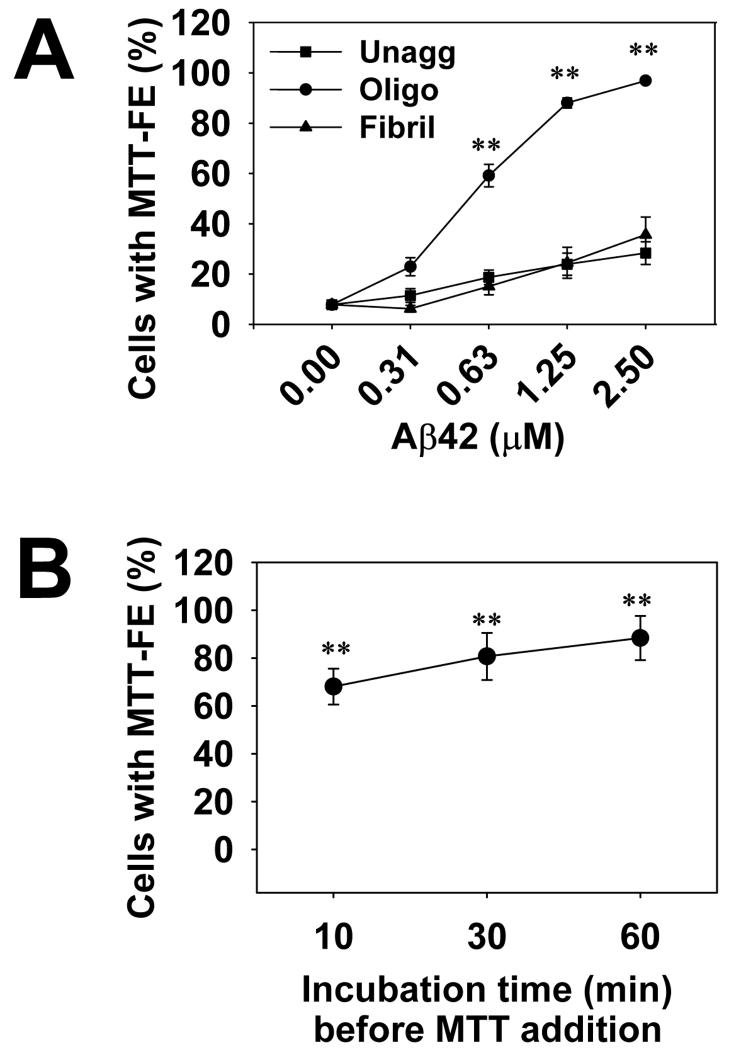
Cells were treated with three preparations of Aβ42: unaggregated, oligomeric, and fibrillar forms. At various time points after Aβ addition, MTT was added and the cultures were further incubated for 1 hr. Cultures were then photographed and cells with surface MTT formazan crystals were quantified. We found that, by restricting the incubation time to under 2 hrs (including 1 hr for MTT incubation) using a protocol described under Experimental Procedures, Aβ42 oligomers induced a significant, concentration-dependent increase in MTT-FE in up to 100% of cells, whereas unaggregated and fibrillar Aβ induced much smaller degrees of MTT-FE (Fig. 1A). The small degree of MTT-FE induced by unaggregated Aβ at higher concentrations was possibly related to Aβ oligomers that were invariably formed during incubation, as demonstrated by size exclusion chromatography and electron microscopy (data not shown). Although failed to induce significant MTT-FE in the first 2 hrs, Aβ fibril samples, after prolonged incubation (over 12 hrs), eventually induced the same degree of MTT-FE as oligomers, possibly reflecting their weaker toxicity (Liu and Schubert, 1997).
Fig. 1.
Aβ42 oligomers induced rapid MTT-FE in a concentration-dependent (A) and time-dependent (B) manner. The 2-hr MTT-FE protocol (see Experimental procedures) was performed and the percentage of cells showing MTT-FE was counted. Data presented are mean percentages (n = 9 in A and n = 4 in B). Error bars represent standard error. In panel A, N2a cells were treated with indicated concentrations of unaggregated (Unagg), oligomeric (Oligo) and fibrillar (Fibril) Aβ42 samples. Aβ42 oligomers above 0.63 μM induced significantly higher levels of MTT-FE compared to unaggregated or fibrillar Aβ of the same concentrations (** p < 0.001). In panel B, N2a cells were treated with 2.5 μM of Aβ42 oligomers for the indicated periods of time, followed by 1 hr MTT. Incubation with Aβ42 oligomers for as short as 10 min induced a significant increase in MTT-FE, compared to mock treatment with solvent (DMSO) only (** p < 0.001).
Despite these differences, when the total MTT in the 2-hr cultures was solubilized in isopropanol with 0.01%HCl (a procedure widely used to quantify cell viability in the conventional MTT assay), no difference between the cultures was shown. This lack of apparent cell death was further supported by measuring lactate dehydrogenase levels in the conditioned media or by the LIVE/DEATH assay (data not shown). Therefore the early MTT-FE induced by oligomers represents a form of toxicity conducive to, but not equating to cell death. Taken together, with the 2-hr protocol, MTT-FE can be used to detect an early toxicity of Aβ oligomers at relatively low concentrations (in the neighborhood of 1 μM). This toxicity cannot be detected using the conventional MTT method measuring total MTT levels. The test can be further shortened because MTT-FE could be significantly enhanced by incubations with Aβ42 oligomers for as short as 10 min before the addition of MTT (Fig. 1B).
2.2. Differential solubilization of intracellular MTT and exocytosed MTT
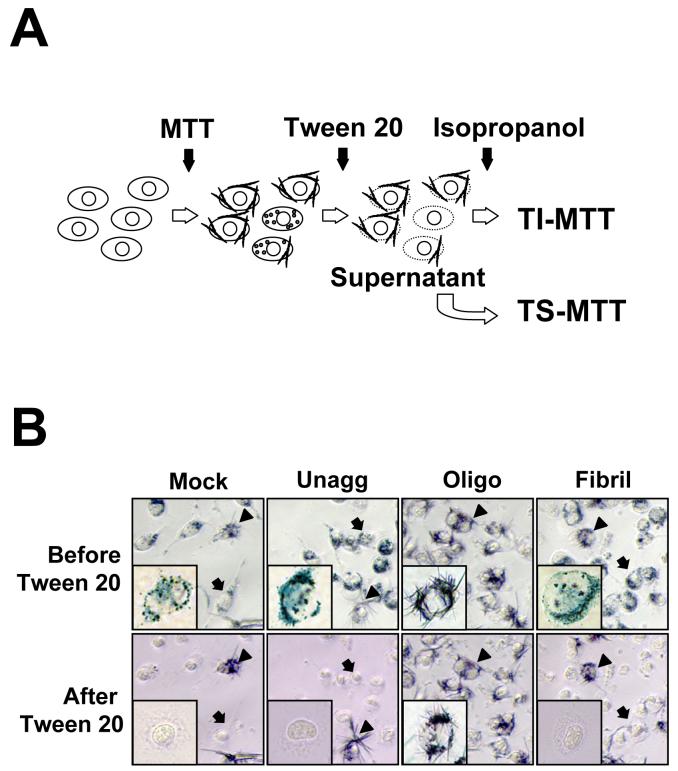
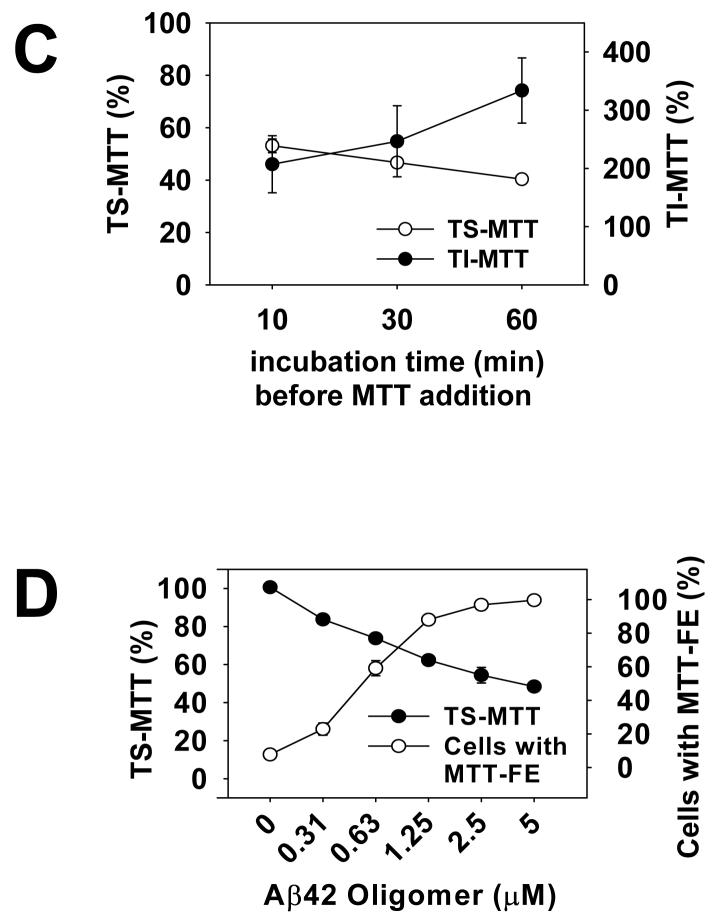
An implication of our results is the possibility of using the short MTT-FE assay for high throughput screening of compounds targeting Aβ oligomers. Although cells forming needle like MTT formazan at the surface after oligomer treatment are clearly distinguishable from those with intracellular, unexcytosed MTT granules, the quantification of cell toxicity by cell counting is time consuming. In addition, cells with co-existing exocytosed MTT and intracellular MTT granules, representing those with intermediate degrees of damage, were frequent and ambiguous for classification. In order to develop a high throughput method that accurately measures the degree of cytotoxicity, we tested whether the intracellular MTT granules and the cell surface MTT crystals can be differentially solubilized and thus quantifiable separately. Our method is schematically presented in Fig. 2A. We found that 1% Tween 20 completely solubilized the intracellular granular MTT formazan, but left the exocytosed needle like MTT formazan intact at the surface of remaining cellular skeleton (Fig. 2B). These insoluble crystals were then dissolved completely by isopropanol. We measured the absorbance of Tween-20 soluble MTT (TS-MTT) and Tween-20 insoluble MTT (TI-MTT), respectively, at 590 nm using a plate reader. A time course experiment showed an inverse relationship between TS- and TI-MTT (Fig. 2C). Exposures to Aβ42 oligomers resulted in progressively lower levels of TS-MTT and progressively higher levels of TIMTT. The values of absorbance obtained from the TI-MTT correlated well with the values obtained from counting cells showing MTT-FE (comparing Fig. 1B and Fig. 2C). Since we repeatedly observed the inverse relationship between TS-MTT and TI-MTT, we used the reduction of TS-MTT to represent Aβ42 oligomer-induced toxicity. As shown in Fig. 2D, there was an oligomer concentration-dependent reduction of TS-MTT, the values of which were inversely related to percentages of cells showing MTT-FE. These results support the feasibility of using a simple differential solubilization method to rapidly quantify Aβ oligomer-induced toxicity.
Fig. 2.
Sequential solubilization of intracellular MTT and exocytosed MTT. The panel A is a schematic of the method. Please see Experimental procedures for a description. The panel B shows photomicrographs of MTT-treated N2a cells, before (upper panel) and after (lower panel) Tween 20 extraction. Prior to the addition of MTT, cells were treated with solvent (Mock), or 2.5 μM unaggregated (Unagg), oligomeric (Oligo), and fibrillar (Fibril) Aβ42, respectively, for one hr. Arrows point to cells with intracellular granules that were completely solubilized by Tween 20 as the TS-MTT. Arrowheads point to cells with cell surface MTT crystals that were not solubilized by Tween 20. Representative cells from each condition are presented in the insets. Most cells in oligomers-treated cultures and a few cells in other cultures showed MTT formazan needles that were not dissolved by Tween 20, but were later dissolved by isopropanol as the TI-MTT. In panel C, N2a cells were treated with 2.5 μM Aβ42 oligomers as described in Fig. 1B. The levels of TS-MTT (empty circles) and TI-MTT (filled circles) were expressed as mean percentages with the mock treatment (DMSO solvent only) group set at 100%. Data presented are means from four independent experiments. Error bars represent standard error. In panel D, parallel sets of cells were treated with indicated concentrations of Aβ42 oligomers and the 2-hr protocol was performed. The levels of TS-MTT (filled circles), expressed as mean percentages with the mock treatment group set at 100%, were measured using one set of cells. Percentages of cells showing MTT-FE (open circles) were counted using the second set of cells. Data presented are means from 3 independent experiments. Error bars represent standard error.
2.3. Known anti-Aβ compounds block MTT-FE
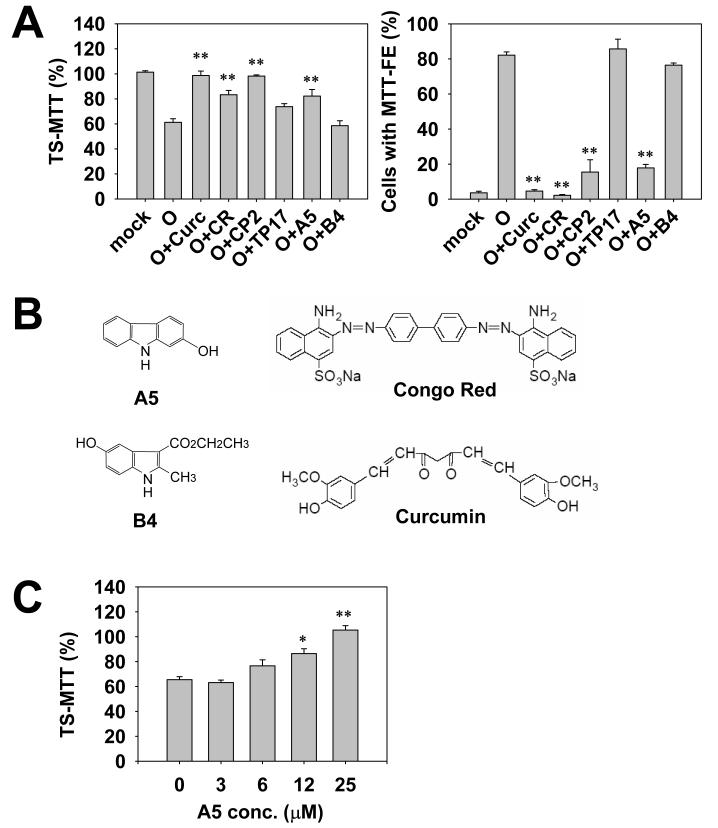
To test the feasibility of the modified MTT-FE assay for identifying anti-Aβ oligomer compounds, Congo red (CR) (Lorenzo and Yankner, 1994; Podlisny et al., 1998; Liu and Schubert, 2006) and curcumin (Yang et al., 2005), compounds known to interact with and affect the aggregation of Aβ, were tested. As predicted, both CR and curcumin successfully inhibited Aβ oligomer-induced MTT-FE and significantly increased TS-MTT (Fig. 3A and 3B).
Fig. 3.
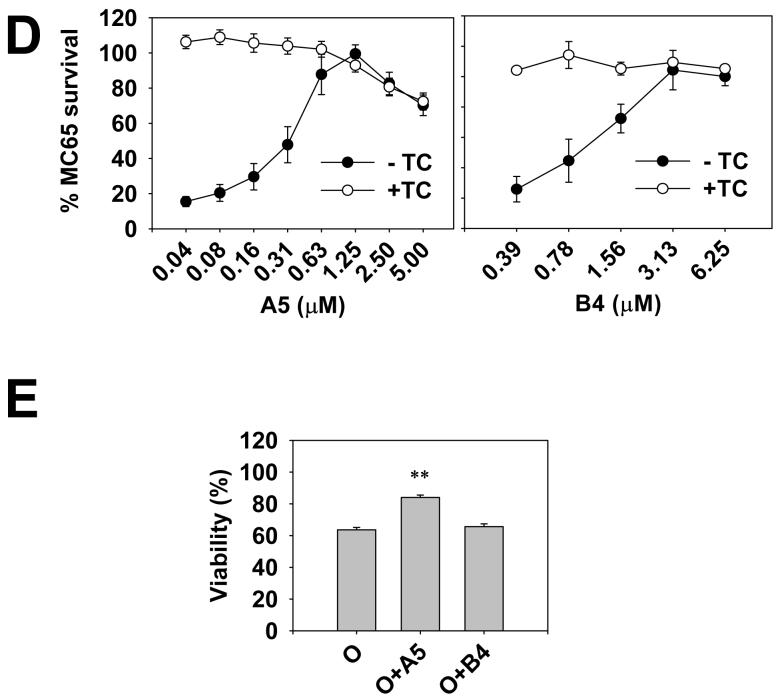
The selection of compounds using the rapid MTT-FE assay and the MC65 protection assay. Panel A shows that CR, curcumin, CP2 and A5 significantly antagonized the effect of oligomers, as demonstrated by the increase in TS-MTT and the decrease in the counts of cells with MTT-FE. Parallel sets of cells were treated with 2.5 μM Aβ42 oligomers in the absence or presence of indicated compounds (25 μM). The 2-hr protocol was performed. The levels of TS-MTT (left panel), expressed as mean percentages with the mock treatment (DMSO solvent only) group set at 100%, were measured using one set of cells. Percentages of cells showing MTT-FE (right panel) were counted using the second set of cells. Data presented are means from three independent experiments. Error bars represent standard error. ** p < 0.001, compared with oligomer treatment only (O) without compounds. Panel B shows the chemical structures of A5, B4, Congo red, and curcumin, which are not drawn to scale. Panel C shows the concentration-dependency of the A5 effect. The assay was performed as described in (A). Data presented are means from three independent experiments. Error bars represent standard error. * p < 0.01, and ** p < 0.001, compared with the no A5 group (0 μM). Panel D shows that both A5 (left panel) and B4 (right panel) protected MC65 cells in a concentration-dependent manner. The cell death program of MC65 cells was initiated by removal of the suppressor tetracycline (TC) to induce the expression of APP-C99 transgene. A5 and B4 of indicated concentrations were added at the same time as TC removal. At 72 hr, viability was assessed by the conventional MTT assay. Data are expressed as mean percentage viability (n = 3) with parallel +TC but no compound cultures set at 100% viability. Error bars represent standard error. Empty circles, +TC; and filled circles, −TC, in the presence of indicated concentrations of compounds. Panel E shows that A5, but not B4, blocked the Aβ42 oligomer-induced death of primary cortical neurons. Neurons (8 days in culture) were treated with 500 nM oligomers (O), or oligomers in the presence of 25 μM of A5 (O+A5) or B4 (O+B4). The mock treatment consisted of solvent (DMSO) only. After 48 hrs, the neuronal viability was measured using the conventional MTT method. Data are expressed as mean percentage viability with mock treatment controls set at 100% viability. Error bars represent standard error. n = 4, ** p < 0.001 compared to the oligomer-treated neurons (O).
We previously identified an anti-Aβ compound code-named CP2 from a focused library of tricyclic pyrones (TPs), using the MC65 protection assay (Jin et al., 2002; Hua et al., 2003; Maezawa et al., 2006). We screened this library using the rapid MTT-FE assay and again identified the lead compound CP2 (Fig. 3A). The ability of CP2 to block the Aβ42 oligomer-induced MTT-FE is consistent with our previous observation that CP2 blocked the toxicity of Aβ42 oligomers to primary neurons and potently protected MC65 cells (Maezawa et al., 2006). TP17, an inactive TP analog (Hua et al., 2003; Maezawa et al., 2006), failed to inhibit MTT-FE (Fig. 3A).
2.4. Selection of candidate compounds from a model combinatorial library
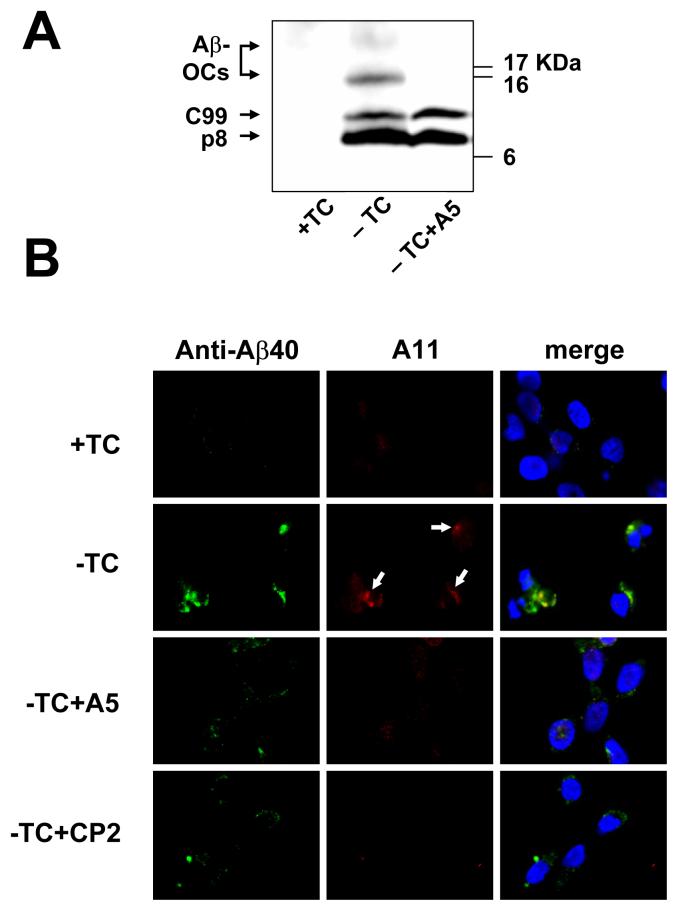
The MC65 is a human neuroblastoma line with conditional expression of APP-C99, which generates Aβ after proteolysis by γ-secretase. In MC65 protection assay, endogenous Aβ production is induced by removal of the transgene suppressor, tetracycline (TC), from the media. Subsequently cells die on day 3 after TC removal (-TC). The death was found to be intimately related to intracellular Aβ oligomer formation, but not related to the small amount of secreted Aβ. Therefore, the MC65 protection assay is a model of neuron death related to intracellular, cell-produced Aβ oligomers (Maezawa et al., 2006). By contrast, in MTT-FE assay, Aβ oligomers are prepared from synthetic Aβ peptides and applied extracellularly. Our observation that both methods selected the same lead CP2 from the tricyclic pyrone library suggests that the selected compound may recognize shared structural features of intracellularly produced and extracellularly applied Aβ oligomers. Supporting this notion, a polyclonal antibody A11 that specifically recognizes the conformation of synthetic Aβ oligomers (Kayed et al., 2003) also positively stained the Aβ40 immunoreactive deposits in –TC MC65 cells (Fig. 4B).
Fig. 4.
Effect of A5 on intacellular Aβ oligomers. In panel A, the Western blot of MC65 cell extracts shows that A5 treatment resulted in a significant reduction of Aβ-OCs. The cytotoxicity program of MC65 cells was initiated by removal of TC in the presence or absence of A5 (1 μM). After 48 hrs, cells were homogenized. Cellular proteins (20 μg protein each) were subjected to Tris/Tricine SDS-PAGE and Western blot analysis with antibody 6E10 (for Aβ1-17). –TC MC65 cells expressed APP-C99, p8 (possibly containing CTFΔ31 and Aβ dimers, see Maezawa et al., 2006), and two major Aβ-OCs of MW 16.5 and 25 kDa, the locations of which are indicated. By contrast, cells cultured in the presence of TC (+TC) showed no expression of Aβ containing proteins derived from APP-C99. Panel B shows that A11, a specific amyloid oligomer antibody, stained a portion of Aβ40-immunoreactive intracellular deposits (arrows) and that these deposits were diminished by A5 and CP2 treatment. MC65 cells, treated as indicated (the concentration of A5 or CP2 was 1 μM) for 36 hrs, were co-stained with Aβ40 specific antibody (green fluorescence) and A11 (red fluorescence). The nuclei were stained with DAPI (blue fluorescence). Representative photomicrographs are shown.
However, CR and curcumin, despite their selection by the MTT-FE assay, were unable to protect MC65 cells, probably due to their failure to sufficiently penetrate the cells and reach the intracellular Aβ targets (Maezawa et al., 2006, the structure of CR and curcumin is shown in Fig. 3B). Therefore we consider that a combination of the two methods may select compounds targeting both intracellular and extracellular Aβ oligomers and substantially eliminate the false leads incurred by individual tests. To validate this approach, we used the two methods independently to screen a model solution phase small molecule 1-alkylamino-5-aryloxyl-2,4-dinitrobenzene combinatorial library. This library was prepared with a method modified from the previously published procedure for a 1,5-dialkylamino-2,4-dinitrobenzene library (Liu et al., 2000 and supplementary Fig. 1). In this modified method, both phenols and amines were used as building blocks so that some of the library compounds contained biphenyl ethers, an important constituent in the structures of several pharmaceutically important chemical templates. Out of the 9216 compounds screened, six compounds were quickly identified that efficiently blocked MTT-FE and increased TS-MTT. All these six compounds shared a dinitrobenzene core and a carbazole building block (code-named A5, Fig. 3B). (The chemical structures of the identified nitroaminophenyl aryl ethers will be described elsewhere.) This result suggested that A5 alone might also be active. Indeed, A5 alone without the dinitrobenzene scaffold was tested and was found to be equally or more effective than the original 1-alkylamino-5-aryloxyl-2,4-dinitrobenze compounds (Fig. 3A and data not shown). A5 increased the levels of TS-MTT in a dose-dependent fashion (Fig. 3C). By contrast, among others, five compounds containing the dinitrobenzene core and an indole building block code-named B4 as well as B4 itself showed no MTT-FE blockage (Fig. 3A and 3B).
Those six A5-containing compounds selected above as well as A5 alone were also identified by the MC65 protection assay (Fig. 3D). This protection was not due to an anti-oxidant effect (Sopher et al., 1996, Maezawa et al., 2004). A5 has a 1,3-relationship of the hydroxyl and amino functions that does not allow the formation of azaquinone structure and is not likely to be an effective antioxidant (Kumar et al., 2005). Interestingly, despite their failure to block MTT-FE, the five compounds with the indole building block B4 as well as B4 alone were also effective in protecting MC65 cells (Fig. 3D). Different from A5, the 1,4-relationship of the hydroxyl and amino functions of B4 (Fig. 3B) likely provides antioxidation and antiinflammatory properties, which might underlie its MC65 protection. Indeed, the HT-22 oxidative stress assay (Tan et al., 1998) showed significant protection offered by B4, but no protective effect by A5 (data not shown).
We further tested whether compounds selected above would have neuroprotective effect on non-neoplastic neurons in culture. We previously demonstrated that CP2 blocked the Aβ oligomer-induced toxicity to primary cultures of cortical neurons (Maezawa et al., 2006), using the conventional MTT method to quantify neuronal viability after a two-day Aβ treatment. Following the same method, we found that A5, but not B4, blocked Aβ oligomer-induced neuronal death (Fig. 3E), consistent with the MTT-FE result. This result supports the ability of the modified MTT-FE assay, in combination with the MC65 protection assay, to select neuroprotective agents.
2.5. CP2 and A5 reduced the level of intracellular Aβ oligomers
We previously showed that the death of MC65 cells is intimately related to intracellular accumulation of Aβ-containing oligomeric complexes (Aβ-OCs) as well as Aβ40-immunoreactive deposits (Maezawa et al., 2006). Fig. 4B demonstrates that the intracellular deposits in –TC MC65 cells contain A11-immunoreactive Aβ oligomers, which may correspond to Aβ-OCs recognized in Western blots (Fig. 4A). CP2, a prototype anti-Aβ aggregation compound that protects MC65 cells, is able to reduce the levels of Aβ-OCs (Maezawa et al., 2006). A5 treatment also substantially reduced the levels of Aβ-OCs (Fig. 4A), which were represented by two major bands of 16.5 and 25 kDa. (Maezawa et al., 2004; Maezawa et al., 2006). Immunocytochemical studies also demonstrated that both Aβ40- and A11- immunoreactive deposits were substantially diminished by CP2 and A5 treatment (Fig. 4B). Taken together, our results suggest that the protective effects of CP2 and A5 are related to their effects on reducing intracellular Aβ oligomers (Maezawa et al., 2006).
2.5. The carbazole A5 blocked the growth of Aβ oligomers
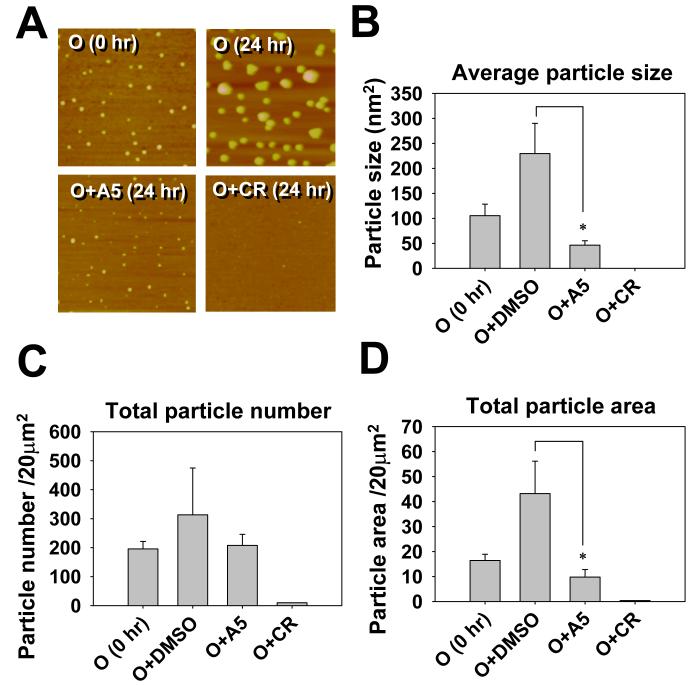
CR, curcumin, and CP2 have been shown to alter the aggregation states of Aβ in aqueous solution after hours to days of incubation (Lorenzo and Yankner, 1994; Podlisny et al., 1998; Yang et al., 2005; Liu and Schubert, 2006, Maezawa et al., 2006). We tested whether A5 might affect the aggregation states of Aβ oligomers by estimating oligomer size using atomic force microscopy (AFM). AFM (Fig. 5A) showed that, upon incubation without inhibitor compounds, Aβ42 oligomers continued to grow to a population with a rather wide size distribution. When incubated in the presence of A5, the growth was not apparent (Fig. 5A, 5B and 5D). The average size of the particles in the A5-treated samples after incubation appeared smaller than the particle size in the pre-incubation sample (0 hr), although this comparison did not reach statistical significance (Fig. 5B). The A5 treatment did not significantly alter the number of particles (Fig. 5C). By contrast, CR substantially disaggregated Aβ oligomers, rendering them almost undetectable by AFM. This result suggests that A5 interacts with Aβ oligomers and halts the growth of oligomers, but is not able to disaggregate Aβ oligomers as CR does.
Fig. 5.
AFM data that demonstrate the ability of A5 to inhibit the growth of existing Aβ42 oligomers. Panel A shows representative 4 μm2 AFM images of Aβ42 oligomer particles. Pre-made Aβ42 oligomers were incubated at 25°C in the absence or presence of 10 equiv. of indicated compounds for 24 hr. DMSO was the solvent of the compounds and was added as the mock control in the sample labeled as O (24 hr). Pictures were taken from the 24-hr samples, except the one labeled as O (0 hr) was from the starting oligomer sample. The average particle size (B), the total particle number (C), and the total area covered by particles (D) were quantified by the Colony program (Fuji Photo Film, Japan). Data presented are mean ± standard error, n = 3, * p < 0.05.
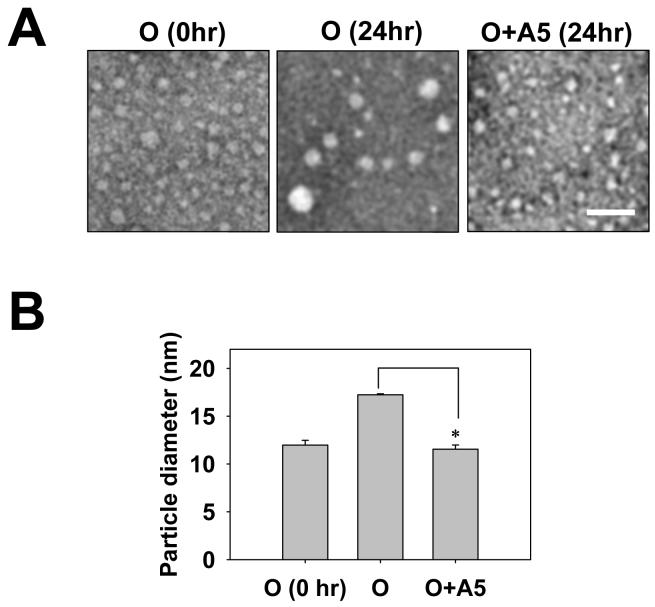
The same samples used for AFM studies were also examined by electron microscopy (EM). As shown in Fig. 6, A5 treatment inhibited the growth of Aβ oligomers, consistent with the AFM data. The same conclusion reached by AFM and EM reduces the possibility that our results were due to A5-altered binding of Aβ oligomers to the AFM mica or EM grids.
Fig. 6.
EM data that corroborate the AFM observation in Fig. 5. Aβ oligomer samples were prepared and named as described in Fig. 5. Representative photomicrographs are shown in (A). Magnification, X50,000; scale bar, 50 nm. Panel B demonstrates the average diameter of particles in each sample. The diameters of particles from randomly selected 250 particles distributed in multiple fields were measured and averaged. Data presented are mean ± standard error from two independent experiments. * p < 0.05.
3. DISCUSSION
Two main conclusions can be drawn from our data. First, Aβ oligomers markedly accelerate the MTT-FE compared to Aβ fibrils or unaggregated Aβ, consistent with previously demonstrated higher cytotoxicity of Aβ oligomers (Dahlgren et al., 2002, Kayed et al., 2004). This property is the basis of our modified MTT-FE assay for rapid compound selection. Second, there is a high concordance between the positive compounds selected by the MC65 protection assay and those by the rapid MTT-FE assay, suggesting the existence of common toxic conformations shared by the intracellular and extracellular forms of Aβ oligomers, which are recognized and blocked by inhibitor compounds. This notion is supported by the detection of their common antigens by the oligomer-specific antibody A11. To our knowledge, MC65 is the first neuron-like line of cells showing intracellular A11-immunoreactive oligomers, which are intimately related to cell death.
The progressive heterogeneity of Aβ assemblies in culture media, seen after prolonged incubations, is minimized by the rapid MTT-FE protocol. Therefore the observed cytotoxicity can be more accurately attributed to oligomers, if the starting material is relatively homogeneous. This is important for the investigation of the structure-toxicity relationship of Aβ, because in principle the protocol can be used to measure the toxicity from a defined aggregation or conformation state of Aβ, if a homogeneous population of Aβ oligomers can be prepared (For example, see Barghorn et al., 2005; Wellnitz et al., 2005; Lesné et al., 2006). A short protocol would also accelerate the compound discovery from a large number of medicinal libraries. Furthermore, the stepwise solubilization and separation of the Tween 20-soluble intracellular MTT granules and Tween 20-insoluble exocytosed MTT makes it possible to accurately measure the Aβ oligomer-induced cytotoxicity in a high throughput and automated fashion.
Although convenient for quickly screening a large number of compounds, our modified MTT-FE assay can occasionally give rise to false positive and false negative results. For example, the MTT-FE assay selected a compound named U18666A, a class 2 amphiphiles that impairs intracellular cholesterol trafficking by a direct inhibition of the function of NPC1 protein (Niemann-Pick type C disease protein 1) (Lange et al., 2000; Jin et al., 2004). U18666A completely blocked MTT-FE, but this effect was due to inhibition of vesicular transport rather than inhibition of Aβ oligomer toxicity (Liu et al., 1997; I. Maezawa and L-W. Jin, unpublished observation). In addition, compounds by themselves may enhance MTT-FE and therefore generate false negative results. Most compounds we have tested so far did not do so; however, we have identified a group of fluorenes that potently enhance MTT-FE (H-S. Hong and L-W. Jin, unpublished observation).
The possibility of false results by the MTT-FE assay adds to the reasons for a second, independent screening by the MC65 protection assay. For example, U18666A, false positively inhibiting MTT-FE, did not protect MC65 cells. Conversely, although the indole B4, due to its anti-oxidant effect, was selected by the MC65 protection assay, it was eliminated by the MTT-FE assay. Therefore, the two assays may complement each other in selecting compounds specifically targeting toxic Aβ oligomers. It is significant that both methods, in our screening of two unrelated libraries, led to the same leads, CP2 and A5, suggesting that both assays likely select for the same anti-Aβ oligomer properties displayed by these compounds, irrespective of the different sites (extracellular vs. intracellular) where oligomers are formed and inflict their toxicities. Compounds of this nature would be advantageous in view of the primary role of intraneuronal Aβ in initiating functional and morphological deficiencies in AD, as implicated in several studies (Wirths et al., 2004; Billings et al., 2005; Gouras et al., 2005).
Ample examples show that certain small molecule compounds protect neurons by inhibition or reversal of Aβ aggregation (Lee, 2002; Gestwicki et al., 2004; Liu et al., 2004; Blanchard et al., 2004; Yang et al., 2005; Walsh et al., 2005; Maezawa et al., 2006; Liu and Schubert, 2006). So far most compounds identified by the rapid MTT-FE assay (such as CR, curcumin, CP2, and A5), excluding the false positivities, are able to affect the aggregation propensity or states of Aβ. However, the compound-induced changes in Aβ aggregation states cannot fully explain the MTT-FE inhibition, because the latter occurs rapidly in our short protocol, while the former effect in general requires prolonged incubation to be detectable. In addition, A5, different from CR, had no detectable effect on the size and number of existing Aβ oligomers. It was previously proposed that a cellular receptor or a membrane-signaling molecule that recognizes the common structure of aggregated amyloid peptides may mediate the alterations of intracellular signal transduction, leading to enhanced MTT-FE (Kayed et al., 2003; Liu and Schubert, 1997). Our results suggest a mechanism by which the inhibitor compounds, via binding to Aβ oligomers, mask the toxic conformation, or induce subtle structural changes of Aβ oligomers, resulting in their decreased interactions with cellular substrates that mediate Aβ toxicity. Although future studies are necessary to verify this mechanism, the demonstration that A5 inhibits the growth of existing Aβ oligomers at least indicates that A5 interacts with Aβ oligomers, consisting with our hypothesis. Similarly, CP2 and curcumin have been shown to interact with Aβ oligomers (Yang et al., 2005; Maezawa et al., 2006). When considering treating AD patients whose brains contain abundant neurotoxic Aβ aggregates, compounds able to instantaneously inhibit the toxicity of existing assemblies by blocking their interactions with cellular substrates would be quite valuable. Importantly, this class of compounds would be more likely identified by our cell-based approaches, but would not be selected by any currently available cell-free screening methods, most of which select for apparent alterations of aggregation states.
Based on our results, we propose that our rapid MTT-FE assay is suitable for the first line screening of a large number of compounds. With a second screening using the MC65 protection assay, this combination is likely to lead to the identification of compounds that show promises in ameliorating toxicities resulting from both intraneuronal and extracellular Aβ oligomers, with few false leads. Furthermore, although cell permeation of the compounds is not required for the inhibition of MTT-FE, it is required for MC65 protection (Maezawa et al., 2006). Since membrane permeability in general confers the ability to cross the blood-brain barrier (Skovronsky et al., 2000), a second screening using MC65 protection is likely to select brain permeable compounds suitable for CNS drug development.
4. Experimental procedures
4.1. Preparation of unaggregated and oligomeric Aβ42 solutions and Aβ42 fibrils
Aβ peptides were purchased from Calbiochem (San Diego, CA). Solutions of seedless, unaggregated Aβ and oligomeric, non-fibrillar Aβ were prepared as we previously described (Maezawa et al., 2006). The resulting oligomers were verified by AFM, EM, and size exclusion chromatography as described (Dahlgren et al., 2002; Chromy et al., 2003; Kayed et al., 2004), aliquoted, and stored at −20°C. As a control, scrambled Aβ peptides did not form oligomers, as expected. Aβ42 fibrils were prepared as previously described (Dahlgren et al., 2002). To ensure reproducibility, in all experiments, the Aβ samples were stored in freezers for less than a month and were frozen and thawed only once.
4.2. Modified MTT-FE assay
Although in our experiments the time course and reagents varied according to experimental needs, here we describe the standard 2-hr protocol for compound testing. N2a cells were plated at a density of 20,000 cells/well in 96-well plates in 100 μl of Opti-MEM (Invitrogen, Carlsbad, CA). After overnight incubation, Aβ42 oligomers and compounds of specified amounts were added to the cultures. After incubation for 1 hr at 37°C, MTT (0.5 mg/ml) was added and the cultures were further incubated for 1 hr. At the conclusion of the assay the cultures were photographed with a Zeiss Axioscop equipped with AxioCam MR digital imaging system (Thornwood, NY). The exocytosed MTT presented as needle-like crystals on the cell surface, clearly distinguishable from the intracellular MTT granules (Liu and Schubert, 2006). The percentage of cells exocytosing MTT formazan was determined by counting 300 cells in multiple fields as described (Liu and Schubert, 1997). For sequential solubilization of MTT formazan, intracellular MTT granules were first solubilized by 1% Tween 20 at 37°C for 10 min with shaking. Solubilized MTT-formazan in the supernatant was transferred to a new plate as the Tween 20-soluble MTT (TS-MTT). The remaining cell surface needle-like crystals were solubilized with 100% Isopropanol as the Tween 20-insoluble MTT (TI-MTT). Absorbance values at 590 nm were determined for each fraction using 630 nm as the reference wavelength. A schematic diagram of the method is presented in Fig. 2A.
4.3. Other cultures
MC65 cell protection assays were performed as previously described (Sopher et al., 1994; Jin et al., 2002; Maezawa et al., 2004; Maezawa et al., 2006). Briefly, MC65 cells were grown in the presence of 1 μg/ml TC and the cell toxicity was induced by the removal of TC to induce APP-C99 transgene expression. To do so, the cells were washed extensively, and plated at a density of 1.2 − 1.5 × 105 cells/cm2 in Opti-MEM (without phenol-red) from Gibco/BRL (Carlsbad, CA) without serum and without TC. The cytotoxicity was determined using a colorimetric MTT assay, the results of which were comparable to data obtained using counts of viable cells based on trypan blue exclusion and the LIVE/DEAD assay. The primary cultures of cortical neurons from newborn mice were performed as previously described (Jin et al., 2004). The preparation of cell homogenates and Western blotting were performed as previously described (Maezawa et al., 2004; Jin et al., 2004). The LIVE-DEATH assay for cell viability was performed using a kit from Invitrogen (Carlsbad, CA)
4.4. Immunocytochemistry
Immunofluorescent staining and double staining of cells were performed according to our published protocol (Jin et al., 2004; Maezawa et al., 2004). For Aβ40 staining, we used an Aβ40 end-specific antibody conjugated with biotin (Calbiochem, 1:1,000, v/v), detected by streptavidin-Alexa 488 (Molecular Probes, 1:3,000, v/v). For oligomer staining, we used A11 (Chemicon, 1:700, v/v), detected by anti-rabbit Alexa 568 (Molecular Probes, 1:3,000, v/v). Cells were incubated in the primary antibodies at 37°C for 30 min, and in the secondary reagents at room temperature for 30 min. Stained cells on coverslips were observed under a Nikon Eclipse E600 microscope and photographed by a digital camera (SPOT RTke, SPOT Diagnostics, Sterling Heights, MI).
4.5. Synthesis of compound libraries
A 9216-member small molecule library with two points of diversity was synthesized according to the schema shown in Supplementary Fig. 1. The focused library of tricyclic pyrones was prepared according to our published procedures (Hua et al., 1997; Hua et al., 2003).
4.6. AFM and EM
Samples of Aβ oligomer solutions were divided for AFM and EM studies. AFM was performed and the particle size and number were quantified as previously described (Wacker et al., 2004; Maezawa et al., 2006). For EM, samples of 3 μl were applied to the charged grid and allowed to settle for 10 seconds. The solution was removed by blotting with a piece of filter paper and the sample washed once with 3 μl of water before stained with a mixture of methyl-amine tungstate (nano-W) (Nanoprobes Inc., Upton, NY) and 1% trehalose. After 2 seconds, staining solution was blotted off with a piece of filter paper and the staining procedure was repeats 3 times. Tobacco mosaic virus (TMV) was added and used for internal control and calibration. The samples were observed by a JEOL1230 electron microscope (JEOL USA Inc., Pleasanton, CA).
4.7. Statistics
We examined the statistical significance of differences between groups by applying one-way analysis of variance (ANOVA) with post-hoc Tukey test or Bonferroni tests, using the SigmaStat 3.1 (Systat Inc. Point Richmond, CA) software program.
Supplementary Material
Acknowledgements
This work was supported by grants from the NIH (AG025500), the UC Davis Health Science Research Fund, the UC Davis M.I.N.D. Institute, the UC Davis Alzheimer's Disease Core Center, the National Science Foundation (CHE-0302122 and CHE-0555341), the American Chemical Society PRF (40345-AC1), Medical Research Council, Foundation of Knowledge Improvement, and Discovery Fund.
Abbreviations
- AD
Alzheimer's disease
- Aβ
amyloid-β protein
- APP-C99
the carboxyl terminal 99-residue of amyloid-β precursor protein
- MTT
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide
- MTT-FE
MTT formazan exocytosis
- TS
Tween 20-soluble
- TI
Tween 20-insoluble
- AFM
atomic force microscopy
- EM
electron microscopy
- Aβ-OCs
oligomeric complexes of Aβ
- TC
tetracycline
- TPs
tricyclic pyrones
- CR
Congo red
- N2a
Neuro-2a neuroblastoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section: #8. Disease-Related Neuroscience
LITERATURE REFERENCES
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hillen H. Globular amyloid β-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer's disease. J. Neurochem. 2005;95:834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Aβ causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Blanchard BJ, Chen A, Rozeboom LM, Stafford KA, Weigele P, Ingram VM. Efficient reversal of Alzheimer's disease fibril formation and elimination of neurotoxicity by a small molecule. Proc. Natl. Acad. Sci. U S A. 2004;101:14326–14332. doi: 10.1073/pnas.0405941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- Chromy BA, Nowak RJ, Lambert MP, Viola KL, Chang L, Velasco PT, Jones BW, Fernandez SJ, Lacor PN, Horowitz P, Finch CE, Krafft GA, Klein WL. Self-assembly of Aβ(1-42) into globular neurotoxins. Biochemistry. 2003;42:12749–12760. doi: 10.1021/bi030029q. [DOI] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine WB, Jr., Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J. Biol. Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Gestwicki JE, Crabtree GR, Graef IA. Harnessing chaperones to generate small-molecule inhibitors of amyloid β aggregation. Science. 2004;306:865–869. doi: 10.1126/science.1101262. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Aβ accumulation and origin of plaques in Alzheimer's disease. Neurobiol. Aging. 2005;26:1235–1244. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hua DH, Chen Y, Sin H-S, Maroto MJ, Robinson PD, Newell SW, Perchellet EM, Ladesich JB, Freeman JA, Perchellet J-P, Chiang PK. A One-Pot Condensation of Pyrones and Enals. Synthesis of 1H,7H-5a,6,8,9-Tetrahydro-1-oxopyrano[4,3-b][1]benzopyrans. J. Org. Chem. 1997;62:6888–6896. [Google Scholar]
- Hua DH, Huang X, Tamura M, Chen Y, Woltkamp M, Jin L-W, Perchellet EM, Perchellet J-P, Chiang PK, Namatame I, Tomoda H. Synthesis and bioactivities of tricyclic pyrones. Tetrahedron. 2003;59:4795–4803. [Google Scholar]
- Jin L-W, Hua DH, Shie FS, Maezawa I, Sopher B, Martin GM. Novel tricyclic pyrone compounds prevent intracellular APP C99-induced cell death. J. Mol. Neurosci. 2002;19:57–61. doi: 10.1007/s12031-002-0011-9. [DOI] [PubMed] [Google Scholar]
- Jin L-W, Maezawa I, Vincent I, Bird T. Intracellular accumulation of amyloidogenic fragments of amyloid-β precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am. J. Pathol. 2004;164:975–985. doi: 10.1016/s0002-9440(10)63185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kayed R, Sokolov Y, Edmonds B, McIntire TM, Milton SC, Hall JE, Glabe CG. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J. Biol. Chem. 2004;279:46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- Kumar R, Ramachandran U, Srinivasan K, Ramarao P, Raichur S, Chakrabarti R. Design, synthesis and evaluation of carbazole derivatives as PPARα/γ dual agonists and antioxidants. Bioorg. Med. Chem. 2005;13:4279–4290. doi: 10.1016/j.bmc.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J. Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange Y, Ye J, Rigney M, Steck T. Cholesterol movement in Niemann-Pick type C cells and in cells treated with amphiphiles. J Biol. Chem. 2000;275:17468–17475. doi: 10.1074/jbc.M000875200. [DOI] [PubMed] [Google Scholar]
- Lee VM. Amyloid binding ligands as Alzheimer's disease therapies. Neurobiol. Aging. 2002;23:1039–1042. doi: 10.1016/s0197-4580(02)00121-5. [DOI] [PubMed] [Google Scholar]
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Liu G, Fan Y, Carlson JR, Zhao ZG, Lam KS. Solution-phase synthesis of a 1,5-dialkylamino-2,4-dinitrobenzene library and the identification of novel antibacterial compounds from this library. J. Comb. Chem. 2000;2:467–474. doi: 10.1021/cc000016l. [DOI] [PubMed] [Google Scholar]
- Liu Y, Schubert D. Cytotoxic amyloid peptides inhibit cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction by enhancing MTT formazan exocytosis. J. Neurochem. 1997;69:2285–2893. doi: 10.1046/j.1471-4159.1997.69062285.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J. Neurochem. 1997;69:581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Piasecki D. A cell-based method for the detection of nanomolar concentrations of bioactive amyloid. Anal. Biochem. 2001;289:130–136. doi: 10.1006/abio.2000.4928. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dargusch R, Banh C, Miller CA, Schubert D. Detecting bioactive amyloid beta peptide species in Alzheimer's disease. J. Neurochem. 2004;91:648–656. doi: 10.1111/j.1471-4159.2004.02751.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Schubert D. Treating Alzheimer's disease by inactivating bioactive amyloid Beta Peptide. Curr. Alzheimer Res. 2006;3:129–135. doi: 10.2174/156720506776383077. [DOI] [PubMed] [Google Scholar]
- Lorenzo A, Yankner BA. β-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc. Natl. Acad. Sci. U S A. 1994;91:12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Jin L-W, Woltjer RL, Maeda N, Martin GM, Montine TJ, Montine KS. Apolipoprotein E isoforms and apolipoprotein AI protect from amyloid precursor protein carboxy terminal fragment-mediated cytotoxicity through different mechanisms. J. Neurochem. 2004;91:1312–1321. doi: 10.1111/j.1471-4159.2004.02818.x. [DOI] [PubMed] [Google Scholar]
- Maezawa I, Hong H-S, Wu H-C, Battina SK, Rana S, Iwamoto T, Radke GA, Pettersson E, Martin GM, Hua DH, Jin LW. A Novel tricyclic pyrone compound ameliorates cell death associated with intracellular amyloid-β oligomeric complexes. J. Neurochem. 2006;98:57–67. doi: 10.1111/j.1471-4159.2006.03862.x. [DOI] [PubMed] [Google Scholar]
- Podlisny MB, Walsh DM, Amarante P, Ostaszewski BL, Stimson ER, Maggio JE, Teplow DB, Selkoe DJ. Oligomerization of endogenous and synthetic amyloid β-protein at nanomolar levels in cell culture and stabilization of monomer by Congo red. Biochemistry. 1998;37:3602–3611. doi: 10.1021/bi972029u. [DOI] [PubMed] [Google Scholar]
- Skovronsky DM, Zhang B, Kung MP, Kung HF, Trojanowski JQ, Lee VM. In vivo detection of amyloid plaques in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U S A. 2000;97:7609–7614. doi: 10.1073/pnas.97.13.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopher BL, Fukuchi K, Smith AC, Leppig KA, Furlong CE, Martin GM. Cytotoxicity mediated by conditional expression of a carboxyl-terminal derivative of the β-amyloid precursor protein. Brain Res. Mol. Brain Res. 1994;26:207–217. doi: 10.1016/0169-328x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Sopher BL, Fukuchi K, Kavanagh TJ, Furlong CE, Martin GM. Neurodegenerative mechanisms in Alzheimer disease. A role for oxidative damage in amyloid beta protein precursor-mediated cell death. Mol. Chem. Neuropathol. 1996;29:153–168. doi: 10.1007/BF02814999. [DOI] [PubMed] [Google Scholar]
- Stine WB, Jr., Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-β peptide oligomerization and fibrillogenesis. J. Biol. Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- Takahashi RH, Almeida CG, Kearney PF, Yu F, Lin MT, Milner TA, Gouras GK. Oligomerization of Alzheimer's β-amyloid within processes and synapses of cultured neurons and brain. J. Neurosci. 2004;24:3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J. Cell. Biol. 1998;141:1423–1432. doi: 10.1083/jcb.141.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker JL, Zareie MH, Fong H, Sarikaya M, Muchowski PJ. Hsp70 and Hsp40 attenuate formation of spherical and annular polyglutamine oligomers by partitioning monomer. Nat. Struct. Mol. Biol. 2004;11:1215–1222. doi: 10.1038/nsmb860. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-β oligomers: their production, toxicity and therapeutic inhibition. Biochem. Soc. Trans. 2002;30:552–557. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, Agnaf OE, Hartley DM, Selkoe DJ. Certain inhibitors of synthetic amyloid β-peptide ,Aβ. fibrillogenesis block oligomerization of natural Aβ and thereby rescue long-term potentiation. J. Neurosci. 2005;25:2455–2462. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellnitz S, Friedlein A, Bonanni C, Anquez V, Goepfert F, Loetscher H, Adessi C, Czech C. A 13 kDa carboxy-terminal fragment of ApoE stabilizes Aβ hexamers. J. Neurochem. 2005;94:1351–1360. doi: 10.1111/j.1471-4159.2005.03295.x. [DOI] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Bayer TA. A modified β-amyloid hypothesis: intraneuronal accumulation of the beta-amyloid peptide--the first step of a fatal cascade. J. Neurochem. 2004;91:513–520. doi: 10.1111/j.1471-4159.2004.02737.x. [DOI] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of Aβ oligomers and fibrils and binds plaques and reduces amyloid in vivo. J. Biol. Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.