Abstract
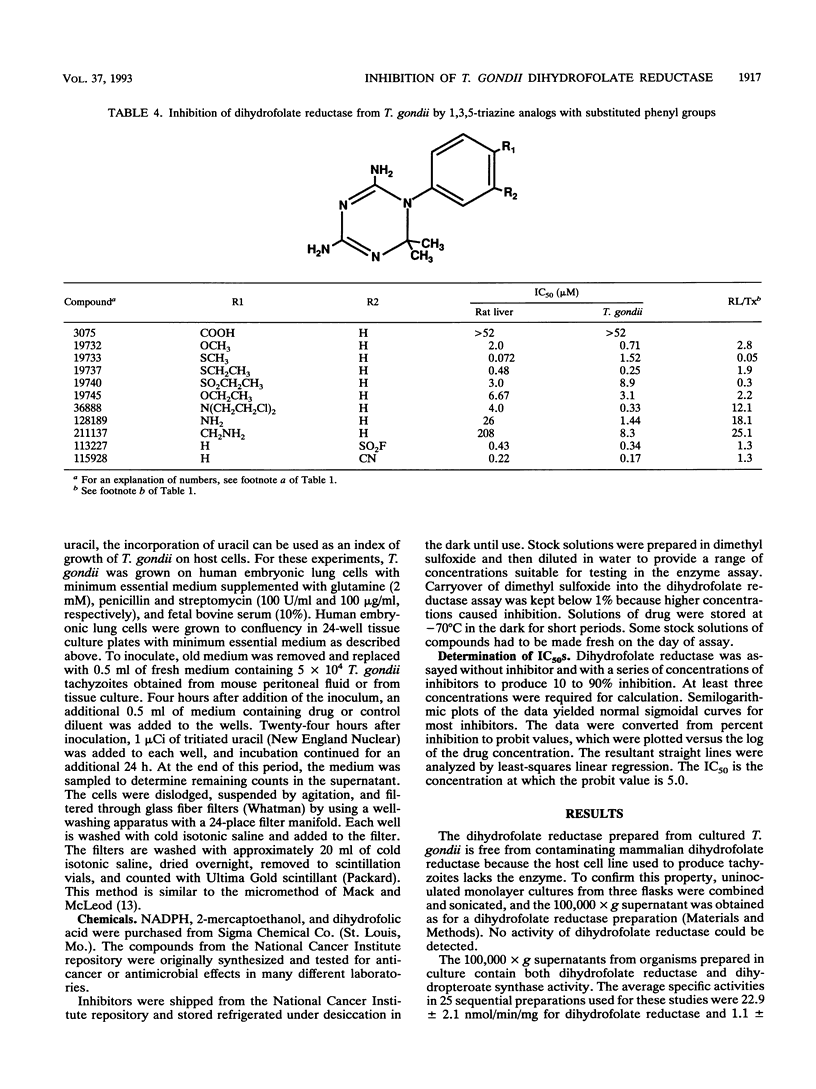
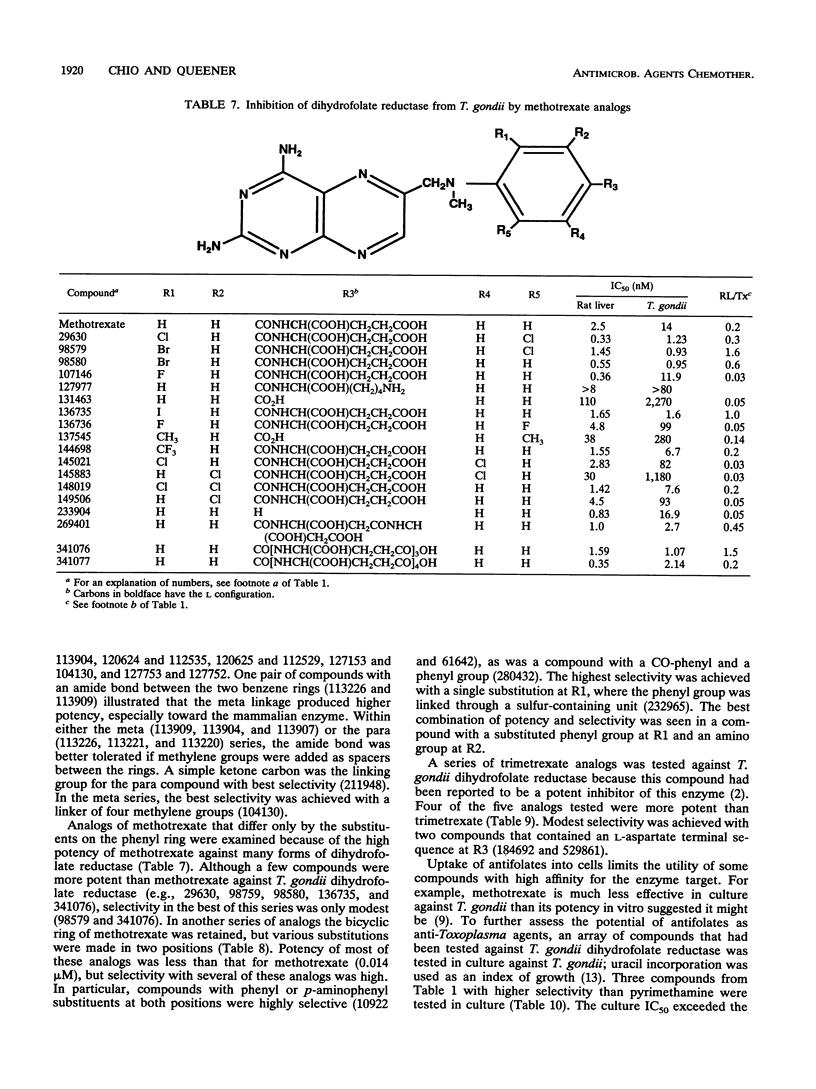
Toxoplasma gondii RH was obtained in high yield from culture in RPMI medium on a line of Chinese hamster ovary cells lacking dihydrofolate reductase activity (ATCC 3952 dhfr-; American Type Culture Collection). Dihydrofolate reductase preparations from harvested organisms had specific activities of 22.9 +/- 2.1 nmol/min/mg. The 50% inhibitory concentrations against reference compounds were 0.014 microM for methotrexate, 0.24 microM for pyrimethamine, 2.7 microM for trimethoprim, and 0.010 microM for trimetrexate. The Km value for NADPH was 11 microM and followed Michaelis-Menten kinetics; the Km for dihydrofolate was ca. 11 microM, but substrate inhibition appeared to occur at high substrate concentrations. Dihydrofolate reductase from T. gondii was used to screen 130 compounds from the National Cancer Institute repository. Thirteen compounds were > 100-fold more potent than pyrimethamine toward T. gondii dihydrofolate reductase; six compounds with various potencies were 8 to 46 times as selective as pyrimethamine for the protozoal form of the enzyme over the mammalian form. Four trimetrexate analogs were more potent than trimetrexate, and two were significantly more selective. Representative compounds were also tested in a culture model of T. gondii employing uracil incorporation as an index of growth. One pyrimethamine analog was as effective as pyrimethamine in inhibiting T. gondii in culture (50% inhibitory concentration, 0.45 microM). Three other compounds were also effective at micromolar concentrations.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegra C. J., Boarman D., Kovacs J. A., Morrison P., Beaver J., Chabner B. A., Masur H. Interaction of sulfonamide and sulfone compounds with Toxoplasma gondii dihydropteroate synthase. J Clin Invest. 1990 Feb;85(2):371–379. doi: 10.1172/JCI114448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra C. J., Kovacs J. A., Drake J. C., Swan J. C., Chabner B. A., Masur H. Potent in vitro and in vivo antitoxoplasma activity of the lipid-soluble antifolate trimetrexate. J Clin Invest. 1987 Feb;79(2):478–482. doi: 10.1172/JCI112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. R. Active-site directed irreversible inhibitors of dihydrofolate reductase. Ann N Y Acad Sci. 1971 Nov 30;186:214–226. doi: 10.1111/j.1749-6632.1971.tb31146.x. [DOI] [PubMed] [Google Scholar]
- Broughton M. C., Queener S. F. Pneumocystis carinii dihydrofolate reductase used to screen potential antipneumocystis drugs. Antimicrob Agents Chemother. 1991 Jul;35(7):1348–1355. doi: 10.1128/aac.35.7.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerman A., Smith H. W., Camerman N. Stereochemistry of dihydrofolate reductase inhibitor antitumor agents: molecular structure of "Baker's antifol" (NSC 139105, triazinate) and "insoluble Baker's antifol" (NSC 113423). Biochem Biophys Res Commun. 1978 Jul 14;83(1):87–93. doi: 10.1016/0006-291x(78)90401-1. [DOI] [PubMed] [Google Scholar]
- Cavallito J. C., Nichol C. A., Brenckman W. D., Jr, Deangelis R. L., Stickney D. R., Simmons W. S., Sigel C. W. Lipid-soluble inhibitors of dihydrofolate reductase. I. Kinetics, tissue distribution, and extent of metabolism of pyrimethamine, metoprine, and etoprine in the rat, dog, and man. Drug Metab Dispos. 1978 May-Jun;6(3):329–337. [PubMed] [Google Scholar]
- Eagon R. G., McManus A. T. Phosphanilic acid inhibits dihydropteroate synthase. Antimicrob Agents Chemother. 1989 Nov;33(11):1936–1938. doi: 10.1128/aac.33.11.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchings G. H., Smith S. L. Dihydrofolate reductases as targets for inhibitors. Adv Enzyme Regul. 1980;18:349–371. doi: 10.1016/0065-2571(80)90025-4. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Allegra C. J., Chabner B. A., Swan J. C., Drake J., Lunde M., Parrillo J. E., Masur H. Potent effect of trimetrexate, a lipid-soluble antifolate, on Toxoplasma gondii. J Infect Dis. 1987 May;155(5):1027–1032. doi: 10.1093/infdis/155.5.1027. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Allegra C. J., Masur H. Characterization of dihydrofolate reductase of Pneumocystis carinii and Toxoplasma gondii. Exp Parasitol. 1990 Jul;71(1):60–68. doi: 10.1016/0014-4894(90)90008-z. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Allegra C. J., Swan J. C., Drake J. C., Parrillo J. E., Chabner B. A., Masur H. Potent antipneumocystis and antitoxoplasma activities of piritrexim, a lipid-soluble antifolate. Antimicrob Agents Chemother. 1988 Apr;32(4):430–433. doi: 10.1128/aac.32.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever O. W., Jr, Bell L. N., McGuire H. M., Ferone R. Monocyclic pteridine analogues. Inhibition of Escherichia coli dihydropteroate synthase by 6-amino-5-nitrosoisocytosines. J Med Chem. 1985 Dec;28(12):1870–1874. doi: 10.1021/jm00150a019. [DOI] [PubMed] [Google Scholar]
- Mack D. G., McLeod R. New micromethod to study the effect of antimicrobial agents on Toxoplasma gondii: comparison of sulfadoxine and sulfadiazine individually and in combination with pyrimethamine and study of clindamycin, metronidazole, and cyclosporin A. Antimicrob Agents Chemother. 1984 Jul;26(1):26–30. doi: 10.1128/aac.26.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer B. I., Dicker A. P., Bertino J. R. Dihydrofolate reductase as a therapeutic target. FASEB J. 1990 May;4(8):2441–2452. doi: 10.1096/fasebj.4.8.2185970. [DOI] [PubMed] [Google Scholar]



