Abstract
Xenopus laevis oocytes are physiologically arrested at G2 of meiosis I. Resumption of meiosis, or oocyte maturation, is triggered by progesterone. Progesterone-induced Xenopus oocyte maturation is mediated via an extranuclear receptor and is independent of gene transcription. The identity of this extranuclear oocyte progesterone receptor (PR), however, has remained a longstanding problem. We have isolated the amphibian homologue of human PR from a Xenopus oocyte cDNA library. The cloned Xenopus progesterone receptor (xPR) functioned in heterologous cells as a progesterone-regulated transcription activator. However, endogenous xPR was excluded from the oocyte nucleus and instead appeared to be a cytosolic protein not associated with any membrane structures. Injection of xPR mRNA into Xenopus oocytes accelerated the progesterone-induced oocyte maturation and reduced the required concentrations of progesterone. In enucleated oocytes, xPR accelerated the progesterone-induced mitogen-activated protein kinase activation. These data suggest that xPR is the long sought after Xenopus oocyte receptor responsible for progesterone-induced oocyte maturation.
Ovarian progesterone is the natural trigger of amphibian oocyte maturation, commonly assessed by germinal vesicle breakdown (GVBD) (1). Masui and Markert (2) demonstrated that progesterone-induced activation of maturation promoting factor (or Cdc2/cyclin B) could occur in enucleated oocytes, thereby establishing the extranuclear nature of the putative progesterone receptor (PR). Studies involving intracellular injection of progesterone and external application of polymer-linked progesterone have suggested that the putative oocyte PR is a membrane-bound, cell surface protein (reviewed in ref. 3). However, many attempts to identify the plasma membrane-bound receptor by classical ligand binding and crosslinking have failed to yield physiologically relevant progesterone-binding proteins in Xenopus oocytes (reviewed in ref. 4).
Although progesterone action in amphibian oocytes is mechanistically distinct from the classical action of progesterone where it regulates transcription via its nuclear PR, no direct evidence is available to rule out the possibility that PR may function in a novel, nongenomic fashion. Furthermore, Sadler and Maller (5) reported that the antiprogestin RU486 could induce oocyte GVBD. RU486 and progesterone interact with distinct, although overlapping regions within the hormone-binding domain (HBD) of PR (6), suggesting that the oocyte PR contains at least the classical progesterone HBD.
In this study we intend to identify the oocyte PR via molecular cloning of the Xenopus genes that contain classical progesterone HBD, using the HBD of human PR as a probe.
Materials and Methods
Molecular Cloning.
cDNA encoding cloned Xenopus progesterone receptor (xPR) amino acids 1–583 (see Fig. 6, which is published as supplementary data on the PNAS web site, www.pnas.org) were subcloned into the expression vector pCS2+MT (7), resulting in an expression plasmid (pCS2+MT-xPR) that encoded five copies of the Myc tag followed by xPR amino acids 1–583. To generate xPR-estrogen receptor (ER) hybrid receptor expression plasmid, we digested pCS2+MT-xPR with BglII (following codon ATC for I339 and XbaI (3′ polylinker region) to remove sequence encoding xPR amino acids 340–583 (roughly corresponding to the HBD at L336-K583). A cDNA fragment corresponding to the human ER HBD (encoding amino acids 282–595) (8) was PCR-amplified with an EcoRI adapter at each end. All ends were blunted before ligation to create pCS2+MT-xPR-ER. A single codon (for Asn) was inserted between xPR I339 and hER S282 as a result of these manipulations.
Oocyte Isolation, mRNA Injection, and Subcellular Fractionation.
General procedures concerning oocyte isolation and mRNA injection have been described (9, 10). For experiments described in Fig. 2B, oocytes were isolated by treating ovarian tissues for 3 h with collagenase solution (9), followed by several washes in OR2 (our standard oocyte incubation medium; 83 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM Na2HPO4, 5 mM Hepes, pH 7.8). The various stages of oocytes were individually selected according to Smith et al. (11), and devitellination (removal of vitellinemembrane) of stage VI occytes was as described (12). Stage VI oocytes were otherwise manually isolated for all other experiments described in this study. Typically, mRNA-injected (or water-injected) oocytes were incubated in OR2 for 24–36 h (unless otherwise indicated) before being subjected to hormonal stimulation or other manipulations.
Figure 2.
Expression of xPR in Xenopus oocytes. (A) Total RNA (1 μg each) from oocytes of the various stages (stages I–III mixed with unknown ratio) were reverse-transcribed followed by PCR amplification using xPR primers encompassing exons E1 and E2. Lane 4 represents a negative control in which PCR was performed directly on input stage VI oocyte RNA without prior reverse transcription (RT). Shown is a representative of four independent experiments with arrows indicating the specifically amplified products. (B) Extracts from stage IV (lane 1), stage V (lane 2), or stage VI (lane 3) oocytes, isolated after collagenase treatment of ovarian tissues, or extracts from collagenase-treated and devitellinated oocytes (lane 4), were immunoblotted with anti-xPR. Equal amounts (50 μg) of proteins were loaded on each lane. The primary antibodies were visualized by incubation with appropriate secondary antibody-horseradish peroxidase conjugates followed by the use of a chemiluminescence kit (ECL, Amersham Pharmacia). Shown is a representative of three independent experiments. (C) Extracts from uninjected (lanes 1, 5, and 9) or Myc-xPR mRNA-injected (lanes 2, 6, and 10) oocytes (each representing half an oocyte), or extracts from untransfected (lanes 3, 7, and 11) or Myc-xPR-transfected (lanes 4, 8, and 12) COS cells (each representing one-tenth of a 3-cm dish) were immunoblotted with the indicated antibodies. Shown is a representative of three independent experiments.
For biochemical isolation of oocyte membranes, 30 oocytes were homogenized (forced through a pipette tip) in 300 μl of ice-cold homogenization buffer (83 mM NaCl/1 mM MgCl2/10 mM Hepes, pH 7.9/0.5 mM PMSF/10 μg/ml leupeptin). The homogenate was clarified by two rounds of low-speed centrifugation (900 × g for 5 min). The clarified supernatant then was subjected to centrifugation at 100,000 × g for 60 min, resulting in total oocyte membrane (pellet) and cytosol (supernatant) (13). Enucleation (11) and isolation of oocyte GV for immunoblotting (14) were performed according to published procedures.
COS Cell Transfection and Related Procedures.
COS cells were seeded on coverslips and either mock-transfected (no DNA control) or transfected with the pCS2+MT-xPR plasmid (xPR) (Lipofectamine, GIBCO). Forty eight hours after transfection, cells were fixed and stained with anti-Myc ascites (1:500) and rhodamine-conjugated second antibodies and visualized by using a confocal microscope.
COS cells (seeded in 23-cm dish) were transfected with mouse mammary tumor virus-chloramphenicol acetyltransferase (CAT) reporter cDNA (250 ng per well), together with one of the various test constructs (vector pCS2+MT, xPR, or xPR-ER, 250 ng DNA per well, unless otherwise indicated). Forty eight hours after transfection, the cells were either left unstimulated (−) or incubated for 18 h with 1 μM (unless otherwise specified) of the following hormones: progesterone, a synthetic progestin (R5020), 17-β estradiol (E2), or dexamethasone. Cells were lysed, and the lysates were subjected to CAT assays according to Prefountaine et al. (15). Quantification of CAT activity was performed by using a PhosphorImager (Bio-Rad).
Anti-xPR and Other Antibodies.
Polyclonal antibodies against xPR were raised by immunizing rabbits with a purified glutathione S-transferase fusion protein containing xPR amino acids 1–215. Antibodies against Xenopus mitogen-activated protein (MAP) kinase (16) and Xenopus nucleolin (R2D2) (14) were gifts of J. A. Cooper (Fred Hutchinson Cancer Research Center, Seattle) and P. J. DiMario (Louisiana State University, Baton Rouge), respectively. Antibodies against Xenopus β-integrin (8C8) were purchased from the Developmental Studies Hybridoma Bank at the University of Iowa. Samples that were destined for anti-β-integrin blotting were dissolved in SDS sample buffer containing no β-mercaptoethanol, because these antibodies do not recognize reduced proteins (17).
Results
Genomic and cDNA Cloning of xPR.
Screening a Xenopus genomic library (a generous gift of M. W. King, Indiana University School of Medicine, Terre Haute) with PCR-amplified human PR HBD (corresponding to amino acids 686–933; ref. 18) as a probe resulted in isolation of a positive clone containing two putative exons, E1 and E2, highly similar to the C terminus of human PR (Fig. 1). A combination of screening an oocyte cDNA library (19) (with E1 and E2 as probe) and performing 5′ rapid amplification of cDNA ends (RACE) resulted in the cloning of a cDNA containing an ORF of 583 aa (Fig. 6). This cDNA contained a putative translation start codon (ACCATGG). However, no in-frame termination codon was detected in the very short upstream sequence (Fig. 6). Repeated attempts by further 5′RACE or PCR experiments have failed to amplify further upstream sequence (not shown). The cloned cDNA contained a putative HBD and a DNA binding domain that are 86% and 92% identical in amino acid sequence to their respective counterparts in human PR. It also contained a nuclear localization signal (286RKFKKFGR). No significant homology was detected between the N-terminal region of this cDNA (encoding amino acids 1–200) and that of human PR; however, significant sequence homology was found with chicken PR (65/200 identity in amino acid sequence, Fig. 7, which is published as supplemental material). Southern analyses using single exons indicated the presence of a single locus in the Xenopus genome (Fig. 8, which is published as supplemental material). Clearly, the cloned cDNA represented the amphibian homologue of mammalian and avian PR and hereafter is referred to as xPR.
Figure 1.
Cloning and sequence analyses of xPR. Schematic representation of xPR sequence as compared with human PR sequence (GenBank accession no. M15716). The introns (line) and exons (E1 and E2) are not drawn in proportion in the approximately 9-kb genomic clone. Other numbers indicate amino acid positions. DBD, DNA binding domain; NSL, nuclear localization signal.
xPR Is Localized Extranuclearly and Appears to be a Cytosolic Protein in Xenopus Oocytes.
Reverse transcription–PCR was carried out to confirm the expression of xPR in Xenopus oocytes. As shown in Fig. 2A, both maturation-incompetent (stage I–IV) and maturation competent (stage VI) oocytes contained xPR mRNA. Polyclonal anti-xPR antibodies detected a prominent band (designated as xPR) of approximately 78 kDa. The size of this protein was slightly greater than the predicted molecular mass of the cloned xPR (66,114). Again, both small (stage IV) and large (stages V and VI) oocytes contained similar levels of xPR protein (Fig. 2B). However, previous studies have suggested that the inability of stage IV oocytes to respond to progesterone was because of other defects distal to the putative PR (20, 21). To eliminate the possibility of any contaminating follicle cells contributing to the xPR protein signal, we removed the vitellinemembrane and hence any attached follicle cells (12). Similar amounts of xPR were detected before (Fig. 2B, lane 3) and after (Fig. 2B, lane 4) the vitellinemembrane was removed. These results established the presence of xPR protein in oocytes.
To further characterize the antibodies and to analyze expression of the cloned cDNA, we injected mRNA encoding Myc-xPR into oocytes and transfected the corresponding cDNA into COS cells. Anti-xPR recognized Myc-tagged xPR (approximately 90 kDa) derived from mRNA injection (Fig. 2C, lane 2) or from cDNA transfection in COS cells (Fig. 2C, lane 4). The identity of Myc-xPR was confirmed by anti-Myc antibodies (Fig. 2C, lanes 6 and 8). The apparent molecular mass difference between the Myc-tagged xPR and endogenous xPR was consistent with the N-terminal addition of five copies of the 13-aa Myc tag. A nonimmune serum did not detect any of these proteins (Fig. 2C, lanes 9–12).
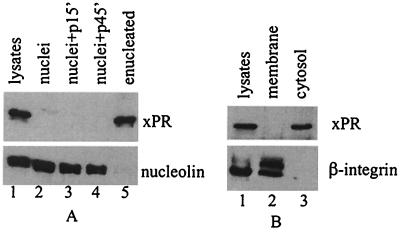
To determine whether endogenous xPR was localized in the nucleus, we carried out enucleation experiments (11, 14). Extracts from the GVs or the enucleated oocytes were analyzed by immunoblotting with anti-xPR. Fig. 3A shows that xPR was retained in the enucleated oocytes (lane 5) but was absent in the GV (lane 2). In contrast, an intranulear protein, nucleolin (14), was recovered in the GV but was undetectable in enucleated oocytes. Incubation of oocytes with progesterone for various periods of time did not cause relocation of xPR to the nucleus (Fig. 3A, lanes 3 and 4). Subcellular fractionation experiments (Fig. 3B) indicated that xPR was not associated with any membrane structures and instead resided in the cytoplasm.
Figure 3.
xPR is a cytosolic protein in Xenopus oocytes. (A) Extracts from intact oocytes (lane 1, equivalent of half an oocyte), isolated nuclei (lane 2, 1 GV), nuclei isolated from oocytes after 15- or 45-min incubation with progesterone (lanes 3 and 4, respectively, 1 GV each), or from enucleated oocytes (lane 5, half an oocyte), were immunoblotted with anti-xPR or anti-Xenopus nucleolin (R2D2) (14). Shown is a representative of five independent experiments. (B) Extracts from intact oocytes (lane 1), 100,000 × g pellet (lane 2), or supernatant (lane 3), each of which were the equivalent of one oocyte, were blotted with anti-xPR or anti-β-integrin. Shown is a representative of five independent experiments.
xPR Functions as a Progesterone-Regulated Transcriptional Activator in COS Cells.
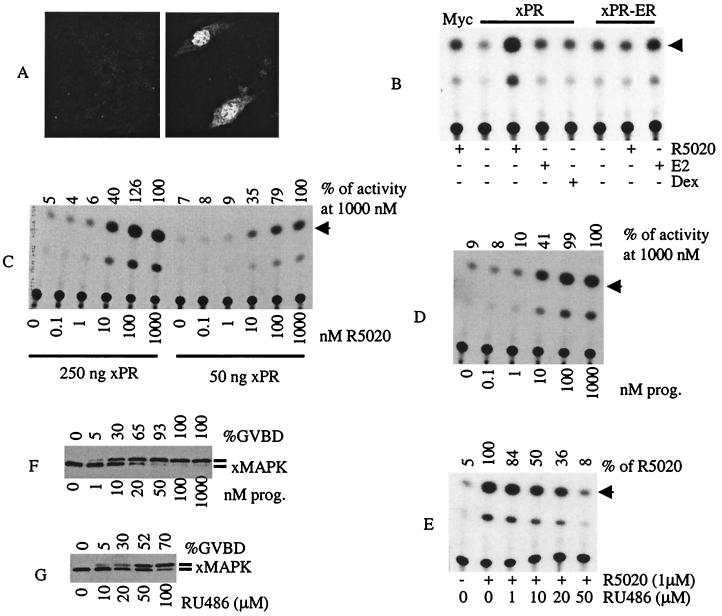
In light of the non-nuclear localization of xPR in oocytes, we wanted to determine whether xPR was capable of functioning as a transcription activator in heterologous cells. In COS cells transiently transfected with xPR cDNA, the expressed protein was detected mainly in the nucleus (Fig. 4A). When cotransfected with a reporter construct containing a progesterone-responsive element (mouse mammary tumor virus-CAT; ref. 15), xPR exhibited progesterone-induced transcriptional activation of the reporter gene (Fig. 4B). In contrast, it did not respond to either 17-β estradiol or dexamethasone. The specificity of xPR for progesterone was further demonstrated by experiments using a hybrid cDNA xPR-ER in which the xPR HBD was replaced with the HBD of human ER. As shown in Fig. 4B, xPR-ER responded to E2 but not to R5020. xPR-ER was a relatively weak transcription factor exhibiting about 2-fold elevation of CAT activity in the presence of E2, compared with the more than 10-fold elevation for xPR in the presence of R5020.
Figure 4.
xPR functions as a transcription activator in COS cells. (A) Typical confocal images of mock-transfected (Left) or Myc-xPR transfected COS cells (Right) immunostained with anti-Myc antibodies. (B) COS cells transfected with mouse mammary tumor virus-CAT, together with the indicated plasmid were stimulated with R5020, 17β estradiol (E2), or dexamethasone (Dex). Cell lysates were prepared and subjected to CAT assays. Shown is a representative of three independent experiments. (C) COS cells transfected with the indicated amounts of xPR plasmid were simulated with the indicated concentrations of R5020. Shown is a representative of three independent experiments. (D) Experiments were similar to C except for using progesterone instead of R5020. (E) xPR-transfected COS cells were treated with R5020 (as indicated) or R5020 together with the indicated concentrations of RU486. Shown is a representative of three independent experiments. (F) Freshly isolated oocytes were treated overnight with the indicated concentrations of progesterone. After scoring each sample for GVBD (expressed as % of total treated oocytes), extracts were prepared and analyzed by xMAP kinase immunoblotting. Shown is a representative of three independent experiments. (G) Experiments similar to F except for using RU486 instead of progesterone.
To estimate the binding affinity of xPR for R5020 or progesterone, we performed the COS cell experiments by using various concentrations of R5020 or progesterone. Fig. 4C shows that approximately 50% activation was achieved at 10 nM of R5020 (or progesterone; Fig. 4D) whereas maximum activation required 100 nM. Similar results were obtained when COS cells were transfected with one-fifth the amount of xPR DNA, with the exception that the absolute amounts of CAT activity were correspondingly less (Fig. 4C). These values matched closely the effective concentrations of progesterone (Fig. 4F) or R5020 (not shown) required for inducing oocyte GVBD or MAP kinase activation. Significantly, these values were also in close agreement with the intraovarian progesterone concentration (estimated at 10 nM) (reviewed in ref. 3).
The progesterone antagonist RU486 is capable of inducing oocyte GVBD (5), presumably by binding the putative oocyte PR. RU486 binds human PR but not chicken PR and this difference has been attributed to a single amino acid (Gly722 in human PR and Cys575 in the corresponding position of chicken PR) within the HBD (22). Inspecting the xPR sequence revealed the presence of a cysteine (Cys372) at the corresponding position, indicating similarity to chicken PR. To examine whether xPR was sensitive to RU486, we treated xPR-transfected COS cells with R5020, RU486, or both. Indeed, RU486, at concentrations ranging from 10 to 50 μM, inhibited hormone-induced transcription by xPR (Fig. 4E) or human PR-A (not shown). RU486 (10–50 μM) alone did not alter basal transcription in COS cells or COS cells transfected with xPR cDNA (not shown). Significantly, the same concentrations of RU486 were also effective in inducing oocyte GVBD and MAP kinase activation (Fig. 4G).
Cloned xPR Enhances Progesterone-Induced GVBD via a Nongenomic Mechanism.
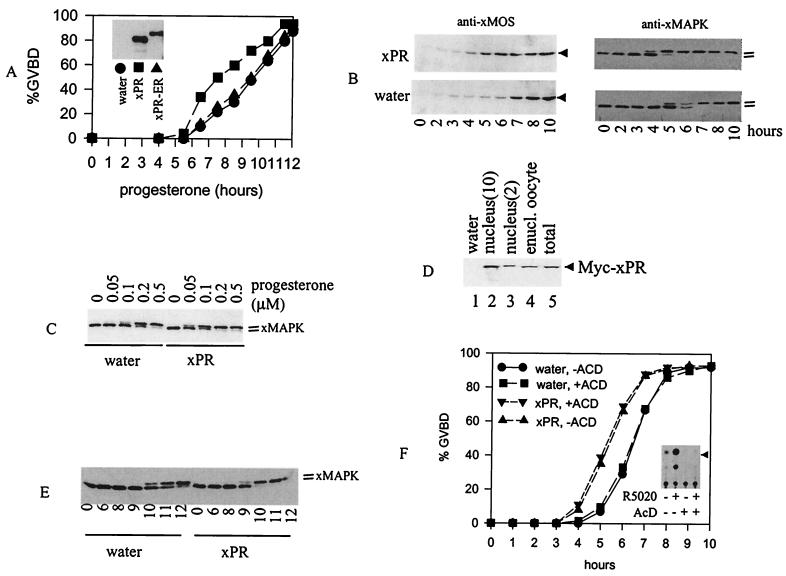
If xPR was indeed responsible for progesterone-induced oocyte maturation, we anticipated that overexpression of xPR in oocytes would potentiate progesterone response. Oocytes injected with mRNA encoding xPR or xPR-ER, or with an equal volume of water, were incubated with progesterone and observed for GVBD. Fig. 5A shows that oocytes injected with xPR mRNA underwent progesterone-induced GVBD more rapidly than oocytes injected with xPR-ER mRNA or water. Because oocytes isolated from different animals tend to vary somewhat in GVBD response time, a more reliable measure of statistical significance among independent experiments would be the acceleration time (time for 50% GVBD in control oocytes minus that in xPR-injected oocytes). In four independent experiments, we obtained acceleration time of 1–3.5 h with a P value of 0.002 by the Student's t test. There were no apparent differences in response time when oocytes injected with xPR-ER mRNA were compared with those injected with water (Fig. 5A). As anticipated, injection of xPR mRNA also accelerated oocyte GVBD when metabolically more stable R5020 (0.5 μM) or RU486 (50 μM) were used (not shown).
Figure 5.
xPR potentiates progesterone-induced MAP kinase activation and GVBD. (A) Oocytes (>60 oocytes per group) injected with water or xPR or xPR-ER mRNA were incubated with 0.5 μM of progesterone. GVBD was scored at the indicated time after the addition of progesterone. Shown is a representative of four independent experiments. (Inset) Typical expression of xPR or xPR-ER in mRNA-injected oocytes as determined via anti-Myc immunoblotting. We estimated that the amount of xPR derived from the injected mRNA was 5–10 times that of endogenous xPR, based on immunoblotting with anti-xPR antibodies (see Fig. 2C). (B) Oocytes injected with water (>200 oocytes) or xPR (>200 oocytes) were treated with progesterone (0.5 μM). At the indicated time, 20–25 oocytes were withdrawn randomly and lysed immediately. All samples were subjected to immunoblotting with anti-xMOS or anti-xMAPK, as indicated. Shown is a representative of three independent experiments. (C) Oocytes injected with water or xPR mRNA were incubated overnight with the indicated concentrations of progesterone and subjected to anti-xMAP kinase immunoblotting. Shown is a representative of three independent experiments. (D) Oocytes injected with water (lane 1) or Myc-xPR mRNA (lanes 2–5) were subjected to nuclear isolation. Extracts from intact oocytes (lanes 1 and 5, one oocyte each), enucleated oocytes (lane 4, one oocyte), or nuclei (lanes 2 and 3, 10 and two nuclei, respectively) were blotted with anti-Myc. Shown is a representative of two independent experiments. (E) Oocytes injected with water (100 oocytes) or xPR (100 oocytes) were individually enucleated (11). The nucleated oocytes were pooled before being divided into six groups of 15 each and treated with progesterone (0.5 μM). At the indicated time after the addition of progesterone, enucleated oocytes were lysed and subjected to immunoblotting with anti-xMAP kinase. Shown is a representative of three independent experiments. (F) Oocytes injected with water, or xPR mRNA, each were split into two groups (>60 oocytes per group) and immediately placed in OR2 or OR2 containing 5 μg/ml of AcD. After a 24-h incubation, progesterone (0.5 μM) was added to all four groups. GVBD was scored at the indicated time after the addition of progesterone. Shown is a representative of two independent experiments. (Inset) CAT assays of xPR-transfected COS cells treated with R5020 (1 μM), AcD (5 μg/ml), alone or in combination.
To further characterize progesterone response in oocytes injected with xPR, we analyzed progesterone-induced synthesis of the germ cell-specific protein kinase MOS (product of the protooncogene c-mos; ref. 23). As expected, progesterone-induced MOS synthesis was significantly accelerated in oocytes injected with xPR compared with oocytes injected with water (Fig. 5B). MOS functions as a MEK kinase in Xenopus oocytes, leading to the ultimate activation of Xenopus MAP kinase (16, 24). We used anti-xMAP kinase immunoblotting to assess the phosphorylation (and hence activation) of xMAP kinase, which corresponded to a slight upward shift in migration (10, 24). As shown in Fig. 5B, activation of xMAP kinase was similarly accelerated in oocytes injected with the xPR mRNA.
We reasoned that an increase of xPR in oocytes via mRNA injection also might reduce the concentrations of progesterone required to elicit a response. Indeed, when suboptimal concentrations (0.05 or 0.1 μM) of progesterone were used, xMAP kinase activation (Fig. 5C) and GVBD (not shown) were significantly enhanced in xPR-injected oocytes. Water- or mRNA-injected oocytes usually were incubated for 24–36 h before the addition of progesterone. Such oocytes required higher concentrations of progesterone to trigger GVBD or MAP kinase activation than freshly isolated oocytes (e.g., Fig. 4F).
Unlike the endogenous xPR (Fig. 3A), which was excluded from the nucleus, xPR derived from mRNA injection was partitioned between the nucleus and enucleated ocoytes (Fig. 5D). Incubation of xPR-injected oocytes with progesterone did not affect the extranuclear/nuclear distribution ratio (not shown). To determine whether the extranuclear xPR was responsible for accelerating progesterone response, we used the enucleation strategy used by Masui and Markert in their seminal study (2) that led to the discovery of cdc2/cyclin B and the establishment of the cytoplasmic nature of the putative oocyte PR. When injected oocytes were subjected to enucleation followed by incubation with progesterone, xMAP kinase activation was accelerated in xPR-injected and enucleated oocytes as compared with water-injected and enucleated oocytes (Fig. 5E). To eliminate the possibility that overexpressed xPR might have caused a genomic (transcriptional) effect before enucleation, we used the general transcriptional inhibitor actinomycin D (AcD). Indeed, inclusion of AcD in the oocyte incubation medium did not affect the ability of xPR to accelerate progesterone-induced GVBD (Fig. 5F). As a control, we showed that the same concentrations of AcD abolished the transcriptional activity of xPR in COS cells (Fig. 5F Inset). These results suggested that the cloned xPR did indeed function in a nongenomic fashion in Xenopus oocytes.
Discussion
In addition to its extranuclear localization (Fig. 3A), which is consistent with a nongenomic role, xPR also appears to fulfill the functional criteria of being the oocyte PR. First, the effective concentrations of progesterone required to induce xPR-dependent transcription in COS cells (Fig. 4 C and D) were the same as those required for inducing GVBD or MAP kinase activation in freshly isolated oocytes (Fig. 4F), suggesting that one and the same protein (xPR) was responsible for both activities. Second, the cloned xPR responded to antiprogestin RU486 in COS cells with effective concentrations (Fig. 4E) that closely mirrored those required to induce GVBD and MAP kinase activation in oocytes (Fig. 4G). Third, injection of xPR mRNA, but not control mRNA (xPR-ER) or water, enhanced the progesterone response (Fig. 5 A–C); and finally a critical functional criterion is its ability to function extranuclearly and independent of transcription (Fig. 5 E and F).
We originally had hoped that injection of xPR-ER mRNA might allow oocytes to undergo E2-dependent maturation. However, this proved unsuccessful despite the presence of xPR-ER fusion protein in the mRNA-injected oocytes (Fig. 5A Inset) and its ability, albeit relatively weak, to respond to E2 in COS cells. The inability of xPR-ER to mediate oocyte maturation suggests the importance of xPR HBD in signaling in addition to its role in hormone binding. This seems likely because hPR HBD is known to contain one of the transcriptional activation domains (AF2) (25).
Although we cannot rule out the possibility that xPR is associated with an intracellular membrane compartment that remains in the supernatant following the high-speed centrifugation, our data (Fig. 3B) clearly indicate that xPR is not associated with the plasma membrane. How do we reconcile the apparent cytoplasmic localization of xPR with previous studies suggesting a membrane-bound, cell surface receptor (reviewed in ref. 4)? First, whether intracellular delivery of progesterone can cause GVBD is controversial (2, 26, 27). We have injected free progesterone ourselves and found that injection of 5 nl of 1 mM progesterone (dissolved in ethanol) per oocyte caused 100% oocytes to undergo GVBD (C. Cummings and X.J.L., unpublished work). We cannot rule out the possibility that progesterone exerted its effect externally after diffusion. Nonetheless, these results argue against the earlier assertion that intracellular delivery of progesterone does not cause GVBD (2, 26) and agreed with a later report (27). Second, it has been reported that cell-impermeable, polymer-linked progesterone (or BSA-linked) is capable of inducing GVBD (28, 29). We have tested BSA-progesterone from Steraloids (Newport, RI) and found that it was equally potent in inducing oocyte GVBD whether by injection or by incubation (C. Cummings and X.J.L., unpublished work). Our results can best be interpreted to mean that the BSA-progesterone preparations Steraloids contain significant levels of free progesterone. Interestingly, a recent report (30) indicates that commercial BSA-E2 preparations (Sigma) contain significant levels of free steroid, which are in fact responsible for the biological activity of these “conjugates.” Furthermore, after removal of free E2 by filtration, the remaining BSA-E2 conjugates do not mimic E2 action at all, indicating that the BSA-linked E2 has actually lost its biological function because of covalent coupling (30). Finally, Sadler and Maller (31) have reported that progesterone is able to inhibit oocyte adenylyl cyclase in partially isolated plasma membrane preparations. Whether these preparations (31) contained at least some xPR (specifically or nonspecifically) remains unknown.
Like progesterone, estrogen is also known to exert both genomic (transcriptional) and nongenomic action. Earlier studies have similarly suggested the existence of a cell surface receptor, based primarily on studies using BSA-E2 or anti-ER antibodies (32). Several recent studies involving reconstitution of the nongenomic action of estrogen, and of progesterone, in COS cells have clearly demonstrated that the classical, intracellular ER, or PR, is also responsible for the nongenomic action (33–35). Intriguingly the nongenomic action of progesterone in these transfected cells appears to require not only PR but also ER, in the form of a heterologous receptor dimer (34). As estrogen is inactive in oocytes (reviewed in ref. 3 and unpublished data), it is unlikely that the progesterone action in oocyte requires ER. Nonetheless all of these studies suggest that classical steroid receptors have dual functions as transcription activators in the nucleus and as signal transducers outside of the nucleus.
Supplementary Material
Acknowledgments
We thank Cathy Cummings for excellent technical assistance. Geoffrey Morris participated in this work as a summer student. We thank the following scientists for reagents: R. Haché, M.-J. Tsai, M. W. King, N. Sagata, J. A. Cooper, and P. J. DiMario. We also thank L. Jaffe, J. Maller, J. M. Baltz, A. Sorisky, G. Prefontaine, and R. Haché for helpful discussions and J. Maller and J. M. Baltz for critical reading of the manuscript. This work is supported by operating grants from the National Cancer Institute of Canada and the National Science and Engineering Research Council of Canada. X.J.L. is a scholar of the Medical Research Council of Canada.
Abbreviations
- GV
germinal vesicle
- GVBD
GV breakdown
- HBD
hormone-binding domain
- MAP
mitogen-activated protein
- PR, progesterone receptor
xPR, Xenopus PR
- ER
estrogen receptor
- AcD
actinomycin D
- RACE
rapid amplification of cDNA ends
- CAT
chloramphenicol acetyltransferase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY007198).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220302597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220302597
References
- 1.Masui Y. J Exp Zool. 1967;166:365–376. doi: 10.1002/jez.1401660309. [DOI] [PubMed] [Google Scholar]
- 2.Masui Y, Markert C L. J Exp Zool. 1971;177:129–146. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 3.Smith L D. Development (Cambridge, UK) 1989;107:685–699. doi: 10.1242/dev.107.4.685. [DOI] [PubMed] [Google Scholar]
- 4.Maller J L. Biol Cell. 1998;90:453–460. [PubMed] [Google Scholar]
- 5.Sadler S E, Maller J L. J Steroid Biochem. 1985;22:419–426. doi: 10.1016/0022-4731(85)90448-0. [DOI] [PubMed] [Google Scholar]
- 6.Vegeta E, Allan G F, Schrader W T, Tsai M J, McDonnell D P, O'Malley B W. Cell. 1992;69:703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- 7.Turner D L, Weintraub H. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- 8.Green S, Walter P, Kumar V, Krust A, Bornert J M, Argos P, Chambon P. Nature (London) 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 9.Liu X J, Sorisky A, Zhu L, Pawson T. Mol Cell Biol. 1995;15:3563–3570. doi: 10.1128/mcb.15.7.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohan N, Agazie Y, Cummings C, Booth R, Bayaa M, Liu X J. J Cell Sci. 1999;112:2177–2184. doi: 10.1242/jcs.112.13.2177. [DOI] [PubMed] [Google Scholar]
- 11.Smith L D, Xu W, Varnold R L. In: Oogenesis and Oocyte Isolation. Kay B K, Peng H B, editors. San Diego: Academic; 1991. pp. 45–60. [DOI] [PubMed] [Google Scholar]
- 12.Methfessel C, Witzemann V, Takahashi T, Michina M, Numa S, Sakmann B. Pflügers Arch. 1986;407:577–586. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- 13.Mora S, Kaliman P, Chillaron J, Testar X, Palacin M, Zorzano A. Biochem J. 1995;311:59–65. doi: 10.1042/bj3110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heine M A, Rankin M L, DiMario P J. Mol Biol Cell. 1993;4:1189–1204. doi: 10.1091/mbc.4.11.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prefontaine G G, Walther R, Giffin W, Lemieux M E, Pope L, Hache R J. J Biol Chem. 1999;274:26713–26719. doi: 10.1074/jbc.274.38.26713. [DOI] [PubMed] [Google Scholar]
- 16.Posada J, Yew N, Ahn N G, Vande Woude G F, Cooper J A. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gawantka V, Ellinger-Ziegelbauer H, Hausen P. Development (Cambridge, UK) 1992;115:595–605. doi: 10.1242/dev.115.2.595. [DOI] [PubMed] [Google Scholar]
- 18.Misrahi M, Atger M, d'Auriol L, Loosfelt H, Meriel C, Fridlansky F, Guiochon-Mantel A, Milgrom E. Biochem Biophys Res Commun. 1987;143:740–748. doi: 10.1016/0006-291x(87)91416-1. [DOI] [PubMed] [Google Scholar]
- 19.Rebagliati M R, Weeks D L, Harvey R P, Melton D A. Cell. 1985;42:769–777. doi: 10.1016/0092-8674(85)90273-9. [DOI] [PubMed] [Google Scholar]
- 20.Sadler S E, Maller J L. Dev Biol. 1983;98:165–172. doi: 10.1016/0012-1606(83)90345-7. [DOI] [PubMed] [Google Scholar]
- 21.Chesnel F, Bonnec G, Tardivel A, Boujard D. Dev Biol. 1997;188:122–133. doi: 10.1006/dbio.1997.8631. [DOI] [PubMed] [Google Scholar]
- 22.Benhamou B, Garcia T, Lerouge T, Vergezac A, Gofflo D, Bigogne C, Chambon P, Gronemeyer H. Science. 1992;255:206–209. doi: 10.1126/science.1372753. [DOI] [PubMed] [Google Scholar]
- 23.Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude G F. Nature (London) 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- 24.Posada J, Cooper J A. Science. 1992;255:212–215. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- 25.Danielian P S, White R, Lees J A, Parker M G. EMBO J. 1992;11:1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith L D, Ecker R E. Dev Biol. 1971;25:233–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- 27.Tso J, Thibier O, Mulner O, Ozon R. Proc Natl Acad Sci USA. 1982;79:5552–5556. doi: 10.1073/pnas.79.18.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godeau J F, Schorderet-Slatkine S, Hubert P, Baulieu E-E. Proc Natl Acad Sci USA. 1978;75:2353–2357. doi: 10.1073/pnas.75.5.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishikawa K, Hanoka Y, Kondo Y, Imai K. Mol Cell Endocrinol. 1977;9:91–100. doi: 10.1016/0303-7207(77)90049-1. [DOI] [PubMed] [Google Scholar]
- 30.Stevis P E, Deecher D C, Suhadolnik L, Mallis L M, Frail D E. Endocrinology. 1999;140:5455–5458. doi: 10.1210/endo.140.11.7247. [DOI] [PubMed] [Google Scholar]
- 31.Sadler S E, Maller J L. J Biol Chem. 1981;256:6368–6373. [PubMed] [Google Scholar]
- 32.Watson C S, Gametchu B. Proc Soc Exp Biol Med. 1999;220:9–19. doi: 10.1046/j.1525-1373.1999.d01-2.x. [DOI] [PubMed] [Google Scholar]
- 33.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. EMBO J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castoria G, Barone M V, Di Domenico M, Bilancio A, Ametrano D, Migliaccio A, Auricchio F. EMBO J. 1999;18:2500–2510. doi: 10.1093/emboj/18.9.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razandi M, Pedram A, Greene G L, Levin E R. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.