Abstract
Recently, Tet Q, a tetracycline resistance determinant that confers resistance by a ribosome protection mechanism, was described and added to the two previously described classes, Tet M and Tet O. The first representative of this class, tetA(Q)1, was isolated from Bacteroides thetaiotaomicron DOT. We report the sequencing of a gene isolated from B. fragilis 1126 which also confers tetracycline resistance. Because of its high degree of identity (97%) with the tetA(Q)1 gene, we defined it as tetA(Q)2. MIC studies revealed that tetA(Q)2 provides a low level of resistance to tetracycline when cloned into Escherichia coli. The extensive homology between tetA(Q)1 and tetA(Q)2 supports the idea of a recent horizontal transfer of tet(Q) genes among Bacteroides spp.
Full text
PDF

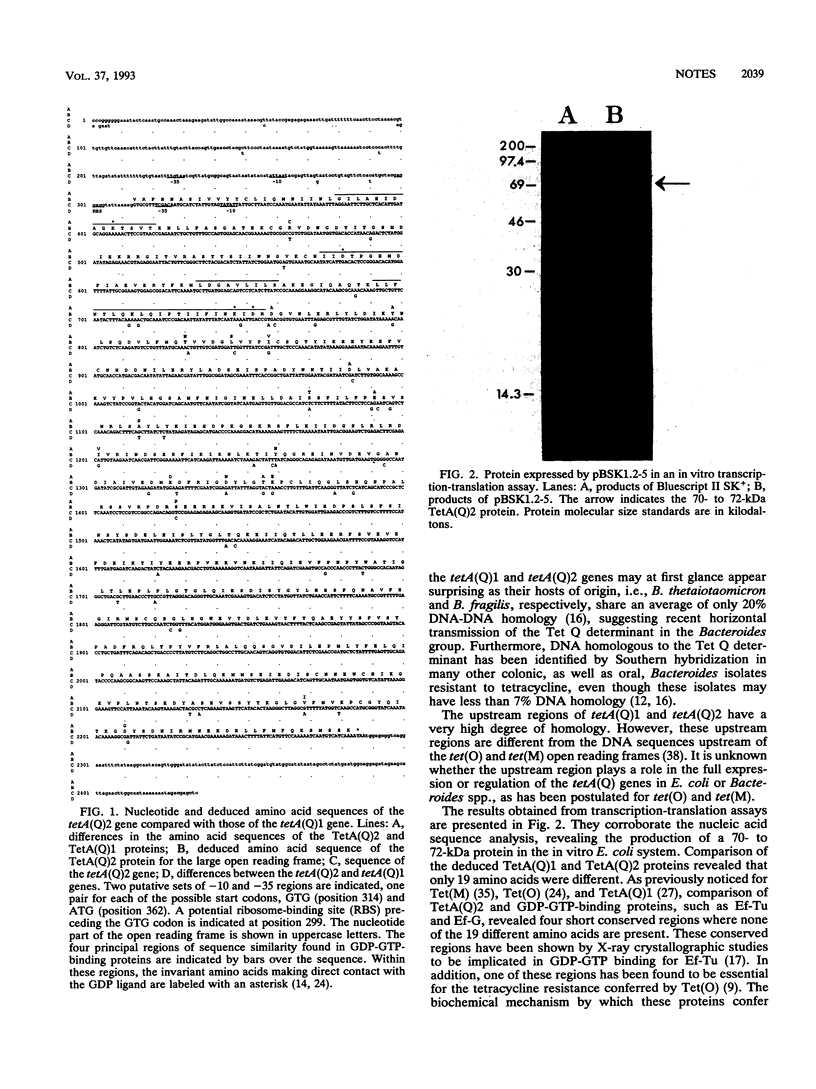


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentorcha F., De Cespédès G., Horaud T. Tetracycline resistance heterogeneity in Enterococcus faecium. Antimicrob Agents Chemother. 1991 May;35(5):808–812. doi: 10.1128/aac.35.5.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V. Nucleotide sequence of the tet(M) gene of Tn916. Nucleic Acids Res. 1990 Oct 25;18(20):6137–6137. doi: 10.1093/nar/18.20.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V. Purification and characterization of Tet(M), a protein that renders ribosomes resistant to tetracycline. J Biol Chem. 1991 Feb 15;266(5):2872–2877. [PubMed] [Google Scholar]
- Clermont D., Horaud T. Identification of chromosomal antibiotic resistance genes in Streptococcus anginosus ("S. milleri"). Antimicrob Agents Chemother. 1990 Sep;34(9):1685–1690. doi: 10.1128/aac.34.9.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald G. F., Clewell D. B. A conjugative transposon (Tn919) in Streptococcus sanguis. Infect Immun. 1985 Feb;47(2):415–420. doi: 10.1128/iai.47.2.415-420.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Bouic K. Detection of conjugal transfer systems in oral, black-pigmented Bacteroides spp. J Bacteriol. 1990 Jan;172(1):495–497. doi: 10.1128/jb.172.1.495-497.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Hasegawa P., Bouic K., Matthews B. Genetic transfer systems in Bacteroides: cloning and mapping of the transferable tetracycline-resistance locus. Mol Microbiol. 1989 Nov;3(11):1617–1623. doi: 10.1111/j.1365-2958.1989.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Guiney D. G., Hasegawa P., Davis C. E. Plasmid transfer from Escherichia coli to Bacteroides fragilis: differential expression of antibiotic resistance phenotypes. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7203–7206. doi: 10.1073/pnas.81.22.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Jr, Hasegawa P., Stalker D., Davis C. E. Genetic analysis of clindamycin resistance in Bacteroides species. J Infect Dis. 1983 Mar;147(3):551–558. doi: 10.1093/infdis/147.3.551. [DOI] [PubMed] [Google Scholar]
- Halliday K. R. Regional homology in GTP-binding proto-oncogene products and elongation factors. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(6):435–448. [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Johnson S. R., Zenilman J. M., Roberts M. C., Morse S. A. High-level tetracycline resistance resulting from TetM in strains of Neisseria spp., Kingella denitrificans, and Eikenella corrodens. Antimicrob Agents Chemother. 1988 May;32(5):765–767. doi: 10.1128/aac.32.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Bouguénec C., de Cespédès G., Horaud T. Presence of chromosomal elements resembling the composite structure Tn3701 in streptococci. J Bacteriol. 1990 Feb;172(2):727–734. doi: 10.1128/jb.172.2.727-734.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N., Titmas B. M., Smith C. J., Tenover F. C. Nucleotide sequence analysis of tetracycline resistance gene tetO from Streptococcus mutans DL5. J Bacteriol. 1988 Aug;170(8):3618–3626. doi: 10.1128/jb.170.8.3618-3626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavathu E. K., Fernandez C. L., Cooperman B. S., Taylor D. E. Molecular studies on the mechanism of tetracycline resistance mediated by Tet(O). Antimicrob Agents Chemother. 1990 Jan;34(1):71–77. doi: 10.1128/aac.34.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavathu E. K., Hiratsuka K., Taylor D. E. Nucleotide sequence analysis and expression of a tetracycline-resistance gene from Campylobacter jejuni. Gene. 1988;62(1):17–26. doi: 10.1016/0378-1119(88)90576-8. [DOI] [PubMed] [Google Scholar]
- Nikolich M. P., Shoemaker N. B., Salyers A. A. A Bacteroides tetracycline resistance gene represents a new class of ribosome protection tetracycline resistance. Antimicrob Agents Chemother. 1992 May;36(5):1005–1012. doi: 10.1128/aac.36.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odelson D. A., Rasmussen J. L., Smith C. J., Macrina F. L. Extrachromosomal systems and gene transmission in anaerobic bacteria. Plasmid. 1987 Mar;17(2):87–109. doi: 10.1016/0147-619x(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Roberts M. C., Brown B. A., Steingrube V. A., Wallace R. J., Jr Genetic basis of tetracycline resistance in Moraxella (Branhamella) catarrhalis. Antimicrob Agents Chemother. 1990 Sep;34(9):1816–1818. doi: 10.1128/aac.34.9.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Hillier S. L. Genetic basis of tetracycline resistance in urogenital bacteria. Antimicrob Agents Chemother. 1990 Feb;34(2):261–264. doi: 10.1128/aac.34.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Moncla B. J. Tetracycline resistance and TetM in oral anaerobic bacteria and Neisseria perflava-N. sicca. Antimicrob Agents Chemother. 1988 Aug;32(8):1271–1273. doi: 10.1128/aac.32.8.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Shoemaker N. B., Guthrie E. P. Recent advances in Bacteroides genetics. Crit Rev Microbiol. 1987;14(1):49–71. doi: 10.3109/10408418709104435. [DOI] [PubMed] [Google Scholar]
- Salyers A. A., Speer B. S., Shoemaker N. B. New perspectives in tetracycline resistance. Mol Microbiol. 1990 Jan;4(1):151–156. doi: 10.1111/j.1365-2958.1990.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Brown J. T., Roberts M., Urdea M. S. The nucleotide sequence of the tetracycline resistance determinant tetM from Ureaplasma urealyticum. Nucleic Acids Res. 1988 Feb 11;16(3):1216–1217. doi: 10.1093/nar/16.3.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Malamy M. H. Sequencing the gene for an imipenem-cefoxitin-hydrolyzing enzyme (CfiA) from Bacteroides fragilis TAL2480 reveals strong similarity between CfiA and Bacillus cereus beta-lactamase II. J Bacteriol. 1990 May;172(5):2584–2593. doi: 10.1128/jb.172.5.2584-2593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Taylor D. E. A DNA sequence upstream of the tet(O) gene is required for full expression of tetracycline resistance. Antimicrob Agents Chemother. 1991 Oct;35(10):2020–2025. doi: 10.1128/aac.35.10.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]