Abstract
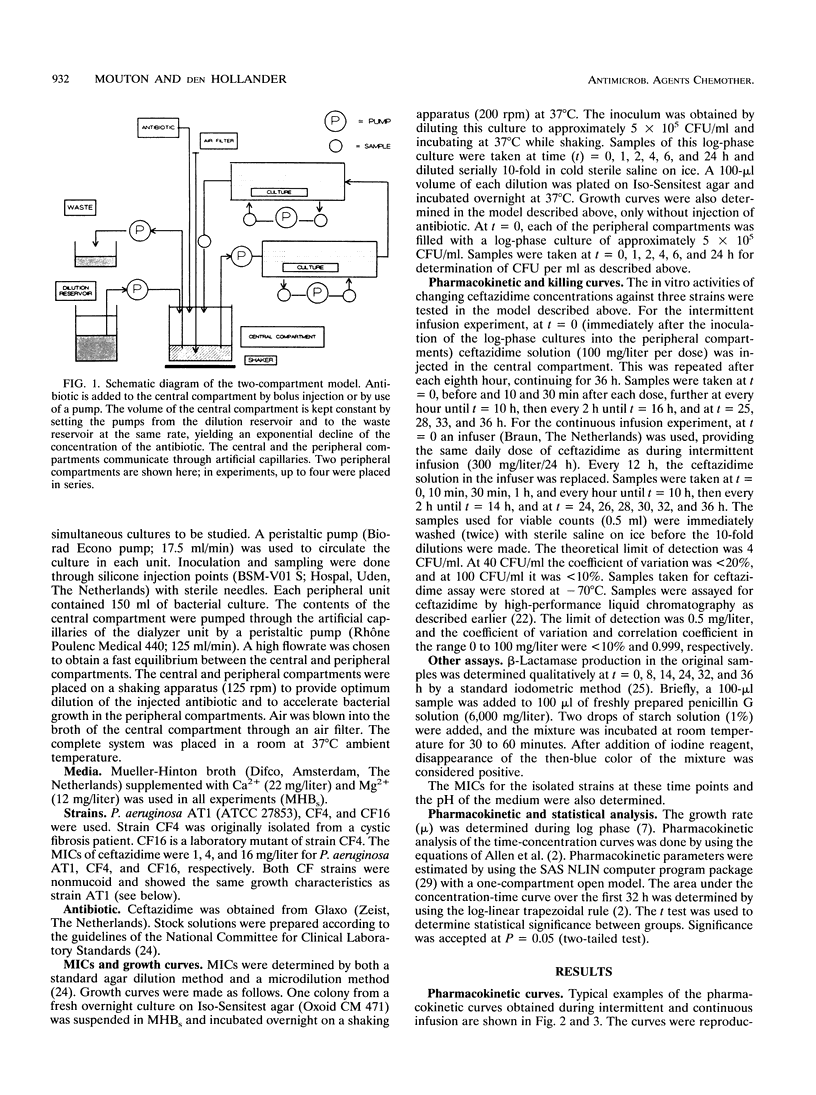
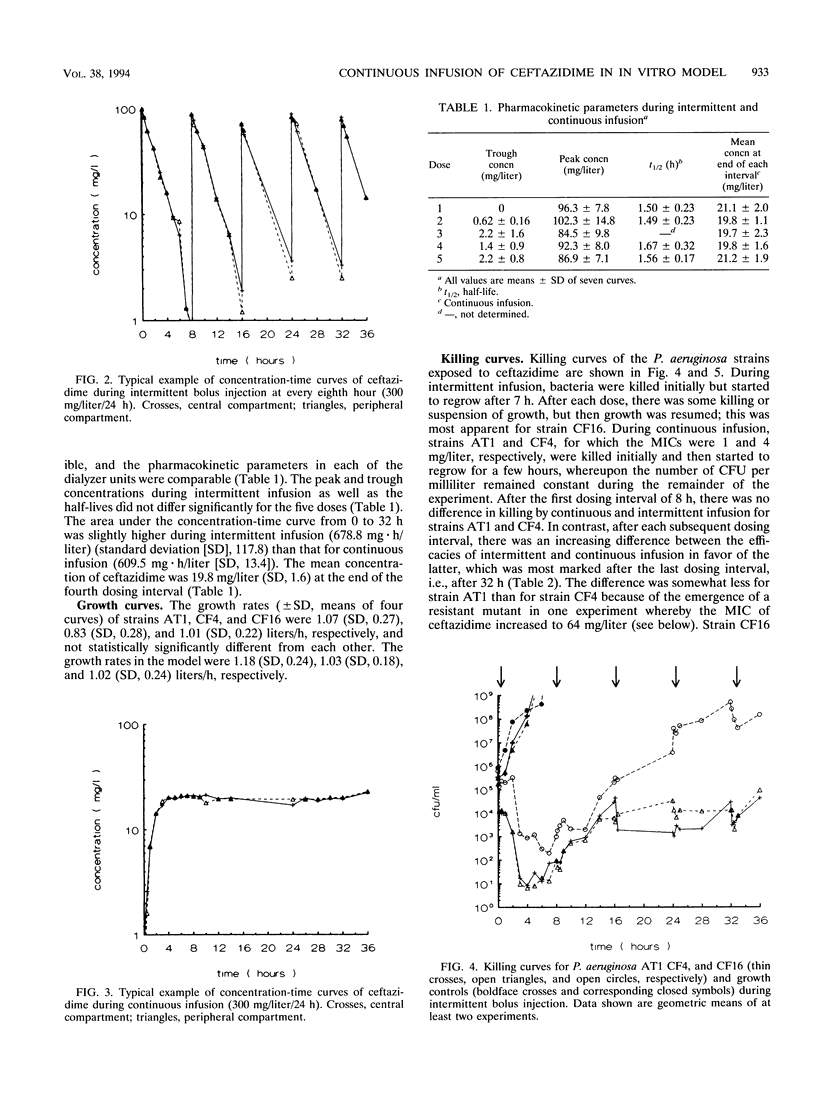
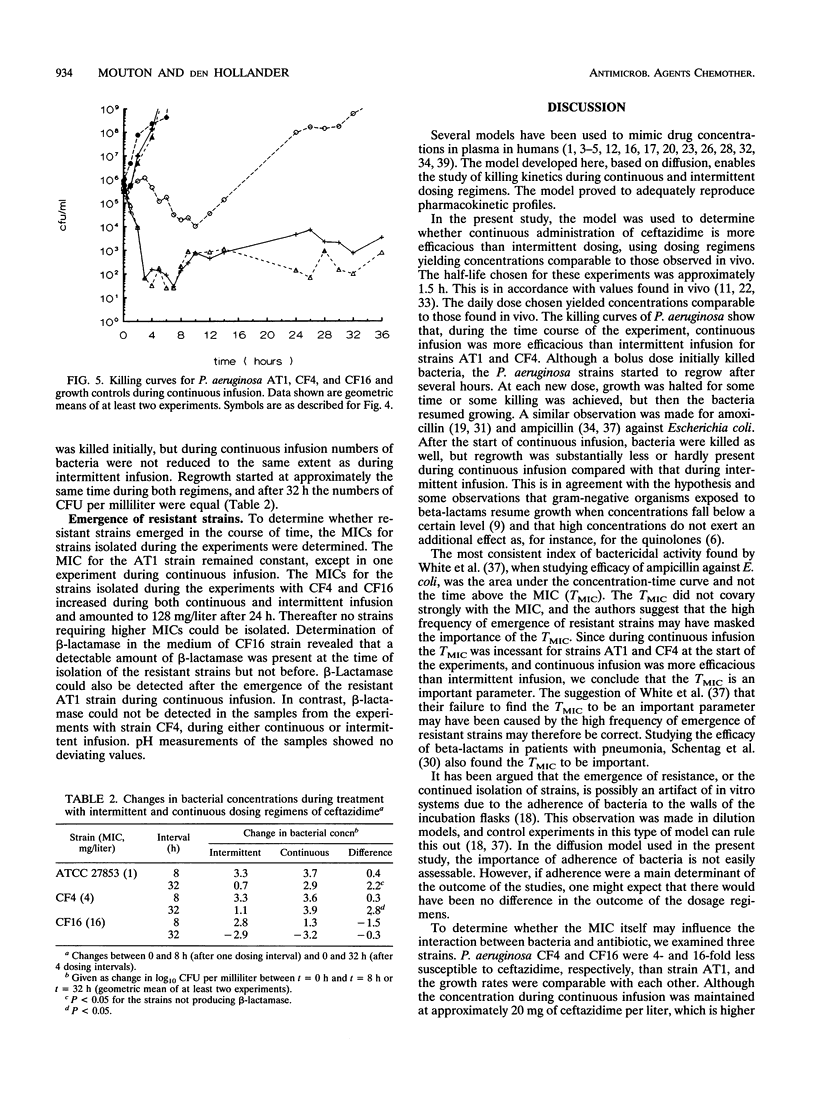
An in vitro pharmacokinetic model mimicking human serum drug concentrations, based on a dialyzer unit, was developed to study the efficacies of continuous infusion and intermittent administration of ceftazidime over a period of 36 h. The daily dose of ceftazidime was 300 mg/liter/24 h given either as a continuous infusion or as three bolus doses. The intermittent dosing regimen yielded peak and trough concentrations after the fourth dose of 92.3 (standard deviation, 8.0) and 1.4 (standard deviation, 0.9) mg/liter, respectively. Continuous administration yielded concentrations of approximately 20 mg/liter. To study efficacy, three Pseudomonas aeruginosa strains, ATCC 27853, CF4, and CF16, were used. The MICs of ceftazidime for these strains were 1, 4, and 16 mg/liter, respectively. Strain CF16 was killed initially during both regimens and then started to regrow. At the end of the fourth dosing interval, i.e., after 32 h, viable counts showed no difference between the regimens. Strains ATCC 27853 and CF4 were killed initially during both dosing schedules, and after the first dosing interval viable counts were similar. However, after the fourth interval, there was a marked difference between bacterial counts during continuous and intermittent infusion, being 2.2 and 2.8 log10, respectively, demonstrating a greater efficacy during continuous infusion. The results indicate that, in the absence of other factors, a sustained level of ceftazidime around or slightly above the MIC is not high enough to maintain efficacy over more than one (8-h) dosing interval. When sustained concentrations higher than four times the MIC are employed, continuous administration in this model is more efficacious than intermittent dosing.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Asadi M. J., Greenwood D., O'Grady F. In vitro model simulating the form of exposure of bacteria to antimicrobial drugs encountered in infection. Antimicrob Agents Chemother. 1979 Jul;16(1):77–80. doi: 10.1128/aac.16.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind A., Jungwirth R., Petermüller C. Simultaneous simulation of the serum profiles of two antibiotics and analysis of the combined effect against a culture of Pseudomonas aeruginosa. Chemotherapy. 1982;28(5):334–340. doi: 10.1159/000238100. [DOI] [PubMed] [Google Scholar]
- Blaser J. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):125–130. doi: 10.1093/jac/15.suppl_a.125. [DOI] [PubMed] [Google Scholar]
- Blaser J., Stone B. B., Groner M. C., Zinner S. H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987 Jul;31(7):1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. A., Ebert S. C. Continuous infusion of beta-lactam antibiotics. Antimicrob Agents Chemother. 1992 Dec;36(12):2577–2583. doi: 10.1128/aac.36.12.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. A., Ebert S. C. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis Suppl. 1990;74:63–70. [PubMed] [Google Scholar]
- Drugeon H. B., Maurisset B., Courtieu A. L. Bactéricidie des aminosides dans un système statique et dans un modèle dynamique. Nouv Presse Med. 1979 Oct 31;8(42):3403–3406. [PubMed] [Google Scholar]
- Gerber A. U., Brugger H. P., Feller C., Stritzko T., Stalder B. Antibiotic therapy of infections due to Pseudomonas aeruginosa in normal and granulocytopenic mice: comparison of murine and human pharmacokinetics. J Infect Dis. 1986 Jan;153(1):90–97. doi: 10.1093/infdis/153.1.90. [DOI] [PubMed] [Google Scholar]
- Gerber A. U., Craig W. A., Brugger H. P., Feller C., Vastola A. P., Brandel J. Impact of dosing intervals on activity of gentamicin and ticarcillin against Pseudomonas aeruginosa in granulocytopenic mice. J Infect Dis. 1983 May;147(5):910–917. doi: 10.1093/infdis/147.5.910. [DOI] [PubMed] [Google Scholar]
- Gerber A. U., Stritzko T., Segessenmann C., Stalder B. Simulation of human pharmacokinetic profiles in mice, and impact on antimicrobial efficacy of netilmicin, ticarcillin and ceftazidime in the peritonitis-septicemia model. Scand J Infect Dis Suppl. 1990;74:195–203. [PubMed] [Google Scholar]
- Gerber A. U., Wiprächtiger P., Stettler-Spichiger U., Lebek G. Constant infusions vs. intermittent doses of gentamicin against Pseudomonas aeruginosa in vitro. J Infect Dis. 1982 Apr;145(4):554–560. doi: 10.1093/infdis/145.4.554. [DOI] [PubMed] [Google Scholar]
- Grasso S., Meinardi G., de Carneri I., Tamassia V. New in vitro model to study the effect of antibiotic concentration and rate of elimination on antibacterial activity. Antimicrob Agents Chemother. 1978 Apr;13(4):570–576. doi: 10.1128/aac.13.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag R., Lexa P., Werkhäuser I. Artifacts in dilution pharmacokinetic models caused by adherent bacteria. Antimicrob Agents Chemother. 1986 May;29(5):765–768. doi: 10.1128/aac.29.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus U., Henninger W., Jacobi P., Wiedemann B. Bacterial elimination and therapeutic effectiveness under different schedules of amoxicillin administration. Chemotherapy. 1981;27(3):200–208. doi: 10.1159/000237978. [DOI] [PubMed] [Google Scholar]
- Leitner F., Goodhines R. A., Buck R. E., Price K. E. Bactericidal activity of cefadroxil, cephalexin, and cephradine in an in vitro pharmacokinetic model. J Antibiot (Tokyo) 1979 Jul;32(7):718–726. doi: 10.7164/antibiotics.32.718. [DOI] [PubMed] [Google Scholar]
- Manual of symbols, equations & definitions in pharmacokinetics. J Clin Pharmacol. 1982 Jul;22(7):1S–23S. [PubMed] [Google Scholar]
- Mordenti J. J., Quintiliani R., Nightingale C. H. Combination antibiotic therapy: comparison of constant infusion and intermittent bolus dosing in an experimental animal model. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):313–321. doi: 10.1093/jac/15.suppl_a.313. [DOI] [PubMed] [Google Scholar]
- Mouton J. W., Horrevorts A. M., Mulder P. G., Prens E. P., Michel M. F. Pharmacokinetics of ceftazidime in serum and suction blister fluid during continuous and intermittent infusions in healthy volunteers. Antimicrob Agents Chemother. 1990 Dec;34(12):2307–2311. doi: 10.1128/aac.34.12.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakawa T., Sakamoto H., Hirose T., Nishida M. New in vitro kinetic model for evaluating bactericidal efficacy of antibiotics. Antimicrob Agents Chemother. 1980 Sep;18(3):377–381. doi: 10.1128/aac.18.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady F., Pennington J. H. Bacterial growth in an in vitro system simulating conditions in the urinary bladder. Br J Exp Pathol. 1966 Apr;47(2):152–157. [PMC free article] [PubMed] [Google Scholar]
- Sanfilippo A., Morvillo E. An experimental model for the study of the antibacterial activity of the sulfonamides. Chemotherapy. 1968;13(1):54–60. doi: 10.1159/000220530. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Smith I. L., Swanson D. J., DeAngelis C., Fracasso J. E., Vari A., Vance J. W. Role for dual individualization with cefmenoxime. Am J Med. 1984 Dec 21;77(6A):43–50. doi: 10.1016/s0002-9343(84)80074-1. [DOI] [PubMed] [Google Scholar]
- Seeberg A. H., Wiedemann B. Application of in-vitro models: development of resistance. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):241–249. doi: 10.1093/jac/15.suppl_a.241. [DOI] [PubMed] [Google Scholar]
- Shah P. M. An improved method to study antibacterial activity of antibiotics in an in vitro model simulating serum levels. Methods Find Exp Clin Pharmacol. 1980 Aug;2(4):171–176. [PubMed] [Google Scholar]
- Sommers D. K., Walters L., Van Wyk M., Harding S. M., Paton A. M., Ayrton J. Pharmacokinetics of ceftazidime in male and female volunteers. Antimicrob Agents Chemother. 1983 Jun;23(6):892–896. doi: 10.1128/aac.23.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjandramaga T. B., Van Hecken A., Mullie A., Verbesselt R., De Schepper P. J., Verbist L. Comparative pharmacokinetics of ceftazidime and moxalactam. Antimicrob Agents Chemother. 1982 Aug;22(2):237–241. doi: 10.1128/aac.22.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toothaker R. D., Welling P. G., Craig W. A. An in vitro model for the study of antibacterial dosage regimen design. J Pharm Sci. 1982 Aug;71(8):861–864. doi: 10.1002/jps.2600710805. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Craig W. A. Kinetics of antimicrobial activity. J Pediatr. 1986 May;108(5 Pt 2):835–840. doi: 10.1016/s0022-3476(86)80754-5. [DOI] [PubMed] [Google Scholar]
- White C. A., Toothaker R. D., Smith A. L., Slattery J. T. Correction for bacterial loss in in vitro dilution models. Antimicrob Agents Chemother. 1987 Nov;31(11):1859–1860. doi: 10.1128/aac.31.11.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. A., Toothaker R. D., Smith A. L., Slattery J. T. In vitro evaluation of the determinants of bactericidal activity of ampicillin dosing regimens against Escherichia coli. Antimicrob Agents Chemother. 1989 Jul;33(7):1046–1051. doi: 10.1128/aac.33.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinner S. H., Husson M., Klastersky J. An artificial capillary in vitro kinetic model of antibiotic bactericidal activity. J Infect Dis. 1981 Dec;144(6):583–587. doi: 10.1093/infdis/144.6.583. [DOI] [PubMed] [Google Scholar]



