Abstract
Most transformed cells display abnormally high levels of RNA polymerase (pol) III transcripts. Although the full significance of this is unclear, it may be fundamental because healthy cells use two key tumor suppressors to restrain pol III activity. We present the first evidence that a pol III transcription factor is overexpressed in tumors. This factor, TFIIIC2, is a histone acetyltransferase that is required for synthesis of most pol III products, including tRNA and 5S rRNA. TFIIIC2 is a complex of five polypeptides, and mRNAs encoding each of these subunits are overexpressed in human ovarian carcinomas; this may explain the elevated TFIIIC2 activity that is found consistently in the tumors. Deregulation in these cancers is unlikely to be a secondary response to rapid proliferation, because there is little or no change in TFIIIC2 mRNA levels when actively cycling cells are compared with growth-arrested cells in culture. Using purified factors, we show that raising the level of TFIIIC2 is sufficient to stimulate pol III transcription in ovarian cell extracts. The data suggest that overexpression of TFIIIC2 contributes to the abnormal abundance of pol III transcripts in ovarian tumors.
Keywords: ovarian cancer, pol III
RNA polymerase (pol) III synthesizes several essential products, including tRNA, 5S rRNA, 7SL RNA, and U6 snRNA (1). It is well established that pol III products are overexpressed in many cell lines transformed by DNA tumor viruses, RNA tumor viruses, or chemical carcinogens (e.g., refs. 2–6). These observations also apply to tumors in vivo (7, 8). Thus, Northern blots showed that 7SL RNA is abnormally abundant in every tumor analyzed, relative to normal tissue from the same patient (8). Furthermore, in situ hybridization of breast, lung, and tongue carcinomas revealed increased levels of pol III transcripts in neoplastic cells relative to the surrounding healthy tissue (7, 8).
To maintain a constant size, a cell must duplicate its components before division. Because most of a cell's dry mass is protein, a high rate of protein synthesis is a prerequisite of rapid growth. Indeed, growth rate is directly proportional to the rate of protein accumulation (9). A 50% reduction in protein synthesis causes cells to withdraw from cycle and quiesce (10, 11). The availability of tRNA and rRNA is clearly an important determinant of the rate of translation. High levels of pol III transcription are therefore necessary to sustain rapid growth. This may help explain the frequent deregulation of pol III in transformed cells. However, pol III is also activated in several slowly growing tumor cell types, such as the osteosarcoma line SAOS2 (12). This shows that the strong correlation between transformation and pol III activation is not simply a consequence of rapid growth.
Although elevated pol III transcript levels are frequently observed in transformed cells, in most cases the mechanistic basis is unknown. A partial explanation was suggested by the discovery that the retinoblastoma protein RB can repress pol III (12–14). Overexpression of RB in vitro or in transfected cells inhibits pol III transcription, whereas specific inactivation of RB in knockout mice causes a 5-fold increase in tRNA and 5S rRNA synthesis (12). This reflects the ability of RB to bind and inactivate the pol III-specific factor TFIIIB (13, 14). RB function is compromised in most human malignancies (15). Several subtle mutations that arose in RB in carcinomas were shown to prevent it from repressing pol III transcription (12). It is therefore likely that the release of TFIIIB from repression by RB will contribute to the activation of pol III transcription in many cancers.
Although unrelated to RB, the tumor suppressor p53 can also bind and repress TFIIIB (16, 17). Overexpression of p53 in vitro or in transfected cells inhibits pol III, whereas specific knockout of p53 allows elevated synthesis of tRNA and 5S rRNA in vivo (16, 17). p53 carries missense mutations in ≈50% of human cancers (18). The effects of such mutations on pol III transcription have yet to be reported, but some may compromise the ability of p53 to control TFIIIB.
TFIIIB has little or no affinity for the majority of pol III templates; in most cases, it is recruited to promoters by protein–protein interactions with the DNA-binding factor TFIIIC2 (1, 19). Situations may therefore arise in which deregulation of TFIIIB has little effect on transcriptional output because TFIIIC2 is limiting. Indeed, TFIIIB activity has been shown to be in relative excess in several mammalian cell types (4, 20, 21). In such situations, activation of TFIIIC2 might have a more profound influence on the rate of pol III transcription. This may explain why adenovirus targets TFIIIC2 for stimulation when it infects HeLa cells (20, 22–24). However, adenovirus also releases TFIIIB from repression by RB, using its E1A oncoprotein (12). Adenovirus therefore ensures rapid pol III transcription in infected cells by targeting two key components of the general class III machinery. A similar situation can occur in transformed cells: TFIIIB is abnormally active in SV40-transformed fibroblasts because the viral large T antigen releases it from the repressive influence of RB (25); in addition, these same cells overexpress TFIIIC2 (4, 25). High levels of pol III transcription follow SV40 transformation; this seems to be achieved by specifically up-regulating both TFIIIB and TFIIIC2 (25).
Although SV40 provides a useful model system for studying transformation, it is not a cause of cancer in humans. It cannot be assumed that studies using cell lines provide an accurate reflection of the situation in real tumors. Substantial differences can exist between the behavior of transformed cells in culture and that of tumors in vivo. For example, only 21% of the genes that are overexpressed in colorectal cancer cell lines are also deregulated in primary colorectal carcinomas (26). For this reason, we have investigated whether TFIIIC2 is overexpressed in tumor biopsy samples relative to healthy tissue isolated in parallel from the same individuals. We used ovarian tumors, which are the leading cause of gynecological cancer deaths in the United States (27). In each ovarian carcinoma examined, we found abnormally elevated TFIIIC2 activity. These observations confirm the relevance of data from SV40-transformed cells and provide the first direct evidence that a pol III transcription factor is deregulated in malignant human cells in vivo.
Materials and Methods
Tissue Specimens.
Ovarian epithelial tumors and adjacent healthy tissue were obtained from Alexandra Hospital, Athens, Greece. The samples were removed surgically and frozen at −70°C. The healthy tissue appeared normal by both macroscopic and microscopic criteria. The tumors were all classified as grade 2 or 3. Tumors 1, 2, and 9 were serous carcinomas; tumors 3, 5, and 8 were mucinous carcinomas; and tumors 4, 6, and 7 were clear cell carcinomas.
Cell Culture.
Cell lines were cultured in DMEM supplemented with 10% FCS/100 units/ml penicillin/100 μg/ml streptomycin. Growth was arrested by transfer to serum-free medium.
RNA Extraction and Reverse Transcription–PCR (RT-PCR).
RNA extraction and reverse transcriptions were performed as previously discussed (25). PCRs were carried out by using a PTC-100 thermal controller (MJ Research, Cambridge, MA). Two microliters of cDNA was amplified with 20 pmol of either tRNATyr primers (5′-CCTTCGATAGCTCAGCTGGTAGAGCGGAGG-3′ and 5′-CGGAATTGAACCAGCGACCTAAGGATGTCC-3′) to give an 84-bp product; 5S rRNA primers (5′-GGCCATACCACCCTGAACGC-3′ and 5′-CAGCACCCGGTATTCCCAGG-3′) to give a 107-bp product; 7SL primers (5′-GTGTCCGCACTAAGTTCGGCATCAATATGG-3′ and 5′-TATTCACAGGCGCGATCCCACTACTGATC-3′) to give a 150-bp product; ARPP P0 primers (5′-GCACTGGAAGTCCAACTACTTC-3′ and 5′-TGAGGTCCTCCTTGGTGAACAC-3′) to give a 265-bp product; TFIIIC220 primers (5′-TCCAGCGAGACCTTCACACC-3′ and 5′-GGATTGAGTGTTGCTGGGCT-3′) to give a 144-bp product; TFIIIC110 primers (5′-CCAGAAGGGGTCTCAAAAGTCC-3′ and 5′-CTTTCTTCAGAGATGTCAAAGG-3′) to give a 303-bp product; TFIIIC102 primers (5′-CCTACTAATGTCCGTTATCTGTGG-3′ and 5′-GCAGAAGTAACATCATTGGC-3′) to give a 184-bp product; TFIIIC90 primers (5′-AAACAGAAGTTGCTGAGTGC-3′ and 5′-ATGGTCAGGCGATTGTCC-3′) to give a 210-bp product; TFIIIC63 primers (5′-CGGCAGATGTTCTACCAGTTATGCG-3′ and 5′-ATGGCTTGAAGTCCTCCTCC-3′) to give a 300-bp product; or GAPDH primers (5′-TCCACCACCCTGTTGCTGTA-3′ and 5′-ACCACAGTCCATGCCATCAC-3′) to give a 452-bp product. Amplification reactions contained 0.5 units of Taq DNA polymerase (Promega) in 20 μl of 1× Taq DNA polymerase buffer (Promega) containing 1.5 mM MgCl2 and 0.2 mM of each dNTP. PCR was performed under the following cycling parameters.
tRNATyr: 95°C for 3 min, 25 or 30 cycles of [95°C for 1 min, 65°C for 30 s, 72°C for 15 s], 72°C for 5 min.
5S rRNA: 94°C for 3 min, 23 cycles of [95°C for 30 s, 58°C for 30 s, 72°C for 1 min], 72°C for 5 min. In this case, 5% DMSO was added to the PCR mix.
7SL RNA: 94°C for 2 min, 20 cycles of [94°C for 1 min, 70°C for 30 s, 72°C for 30 s], 72°C for 2 min.
ARPP P0: 95°C for 2min, 22 or 25 cycles of [95°C for 1 min, 58°C for 30 s, 72°C for 1 min], 72°C for 3 min.
TFIIIC220: 95°C 3 min, 30 cycles of [94°C 20 s, 62°C 30 s, 72°C 30 s], 72°C 10 min.
TFIIIC110: 94°C 3 min, 6 cycles of [95°C 1 min, 66°C 40 s, 72°C 40 s], 22 cycles of [95°C 1 min, 62°C 40 s, 72°C 40 s], 72°C 5 min.
TFIIIC102: 95°C for 3 min, 30 cycles of [95°C for 30 s, 61°C for 30 s, 58°C for 30 s, 50°C for 30 s, 72°C for 1 min], 72°C for 5 min.
TFIIIC90: 95°C for 3 min, 30 cycles of [95°C for 1 min, 55°C for 30 s, 62°C for 1 min, 72°C for 1 min], 72°C for 5 min.
TFIIIC63: 95°C for 3 min, 30 cycles of [95°C for 1 min, 69°C for 1 min, 62°C for 1 min, 50°C for 1 min, 72°C for 1 min], 72°C for 5 min. In this case, 2 mM MgCl2 was used in the PCR mix.
GAPDH: 94°C for 3 min, 18 cycles of [95°C for 30 s, 66°C 40 s, 72°C 1 min], 72°C for 5 min.
Reaction products were resolved on 2% agarose gels and visualized by ethidium bromide staining. Gels were scanned and quantitated by a UVP image analysis system (Adobe photoshop 4.0, Adobe Systems, Mountain View, CA).
Protein Extraction and Fractionation.
To prepare whole cell extracts from frozen tissues, samples were finely minced on dry ice by using a spatula. Protein was then extracted in high salt by using a freeze–thaw procedure, as previously described (28). The same protocol was used to prepare whole cell extracts from the ovarian epithelial cell line ROSE 199 (29).
Protein fractions were prepared from HeLa nuclear extracts by phosphocellulose chromatography, as previously (30). Phosphocellulose B is the 0.1–0.35 M KCl step fraction and contains TFIIIB and pol III; phosphocellulose C is the 0.35–0.6 M KCl step fraction and contains TFIIIC and pol III (30).
TFIIIC2 was immunopurified by using polyclonal antiserum 4286, which was raised by immunizing rabbits with synthetic peptide RPGFSPTSHRLLPTP (human TFIIIC110 residues 897–911) coupled to keyhole limpet hemocyanin. The corresponding preimmune serum was used in control immunopurifications. Sera were purified on protein A-Sepharose before use. HeLa-derived phosphocellulose C fraction (2 ml, 0.7 mg/ml) was incubated on ice with 300 μl of purified antibody (10 mg/ml) for 1 h. This mixture was recirculated slowly through 400 μl of protein A-Sepharose at 4°C for 1.5 h. The column was washed with 50 column volumes of HT buffer [10 mM Tris⋅HCl (pH 7.9)/10% glycerol/5 mM MgCl2/3 mM DTT/0.2 mM PMSF] containing 100 mM NaCl. Immunoaffinity-purified TFIIIC was then eluted by using HT buffer containing 2 M NaCl and 2 M urea. Fractions were dialyzed into LDB buffer (28).
Transcription, Immunoblotting, and Electrophoretic Mobility Shift Assays.
Transcription reactions were carried out as previously described (21). Radiolabeled transcripts were resolved on 7% polyacrylamide sequencing gels and detected by autoradiography. The pVA1 template contains the adenovirus VA1 gene (28).
Immunoblotting was performed as previously described (28) by using monoclonal antibody clone 46 (Transduction Laboratories, Lexington, KY) against TFIIIC110 and antiserum C-18 (Santa Cruz Biotechnology) against TFIIB.
Electrophoretic mobility shift assays (EMSAs) were carried out as previously described (4). The VA1 gene probe was a 129-bp fragment (from −30 to +99) prepared by digestion of pVA1 with XbaI and BstEII. The TFIIIC2 fraction used as a control in Fig. 5A was prepared by sequential chromatography of HeLa nuclear extract on phosphocellulose and heparin-Sepharose, as previously discussed (28).
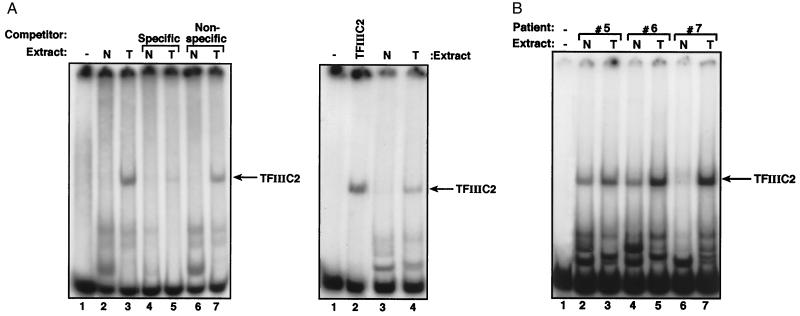
Figure 5.
Ovarian tumors display elevated TFIIIC2 activity. (A Left) EMSA by using 1 ng of VA1 probe, 500 ng of polydI-dC, and no protein (lane 1) or 10 μg of protein extracts of normal ovarian tissue (N, lanes 2, 4 and 6) or ovarian tumor (T, lanes 3, 5 and 7) from patient 7. Lanes 4 and 5 also contain 60 ng of unlabeled VA1 gene fragment. Lanes 6 and 7 also contain 60 ng of unlabeled vector DNA of similar length. (Right) EMSA by using 1 ng of VA1 gene probe, 500 ng of polydI-dC competitor, and no protein (lane 1), 2 μg of partially purified TFIIIC2 (lane 2), or 10 μg of protein extracts of normal ovarian tissue (lane 3) or ovarian tumor (lane 4) from patient 7. (B) EMSA by using 1 ng of VA1 gene probe, 500 ng of polydI-dC competitor, and no protein (lane 1), or 10 μg of protein extracts of normal ovarian tissue (N, lanes 2, 4 and 6) or ovarian tumors (T, lanes 3, 5 and 7) from patients 5, 6, or 7, as indicated.
Results
Pol III Products Are Overexpressed in Ovarian Tumors.
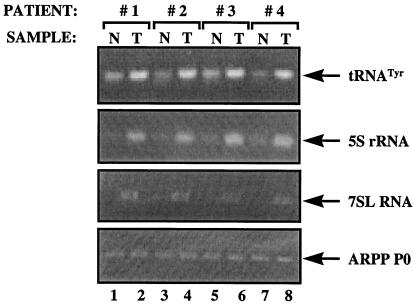
As an indication of the pol III transcriptional activity of tissues at the time of biopsy, we prepared primers that hybridize specifically to the intron sequences of short-lived precursor tRNAs. Because introns are processed from primary transcripts and then degraded very rapidly, their levels in a cell provide an accurate reflection of the rate of ongoing transcription (31). An intron-specific primer was used in RT-PCR reactions to assay the levels of primary transcript from a tRNATyr gene by using as template cDNAs derived from RNA extracted from ovarian tissue samples. The levels of the primary tRNATyr transcript were significantly elevated in each of four ovarian tumor samples when compared with noncancerous tissue isolated in parallel from the same individuals (Fig. 1 Top). This effect was specific, because no change was detected in the levels of mRNA encoding acidic ribosomal phosphoprotein P0 (ARPP P0), which is synthesized by pol II (Fig. 1 Bottom). To assess the generality of the increase observed with tRNATyr, we examined the products of other pol III templates. 5S rRNA and 7SL RNA were found to be expressed at substantially elevated levels in all of the carcinomas (Fig. 1 Middle). This was also true of a tRNAArg (data not shown). Development of ovarian tumors is therefore accompanied by a specific increase in the expression of several genes that are transcribed by pol III. After normalization to the ARPP P0 control, the pol III transcripts were elevated by 2- to 4-fold in the cancers.
Figure 1.
Pol III products are overexpressed in ovarian tumors. cDNAs were generated by reverse transcription of RNA from normal ovarian tissue (N, odd lanes) or ovarian tumors (T, even lanes) from patients 1–4, as indicated. These cDNAs were PCR amplified by using tRNATyr (top panel), 5S rRNA (second panel), 7SL RNA (third panel), and ARPP P0 (bottom panel) primers.
Ovarian Tumors Overexpress TFIIIC2.
As deregulation is observed for a range of class III genes with distinct promotor sequences and structures, it seemed likely that it was mediated through changes in the general pol III transcription machinery that is utilized by each of these templates. Because the general factor TFIIIC2 is activated by SV40 transformation (4, 25), we examined whether TFIIIC2 might also be deregulated in ovarian carcinomas.
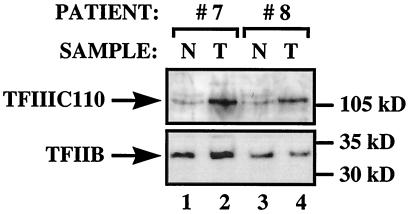
TFIIIC2 is a complex of five polypeptides of 220, 110, 102, 90, and 63 kDa (24, 32). The 220-, 110-, and 90-kDa subunits show histone acetyltransferase activity that may help the factor to gain access to genes that are assembled into chromatin (33). Both adenovirus and SV40 have been shown to induce overproduction of the 110-kDa subunit (24, 25). Immunoblot analysis of biopsy samples from two patients showed that TFIIIC110 is also overexpressed in carcinomas relative to healthy ovarian tissue (Fig. 2). This effect was specific, because there was little or no change in the level of TFIIB.
Figure 2.
Elevated levels of TFIIIC110 polypeptide can be found in ovarian tumors. Proteins (100 μg) extracted from normal ovarian tissue (N, odd lanes) or ovarian tumors (T, even lanes) from patients 7 and 8 were resolved on an SDS-7.8% polyacrylamide gel and then analyzed by Western immunoblotting with antibodies against TFIIIC110 (Upper) and TFIIB (Lower).
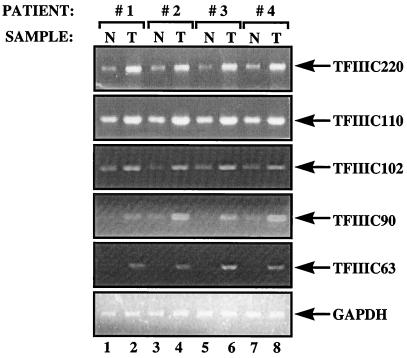
A more extensive analysis was carried out by RT -PCR, to assay levels of the mRNAs encoding each of the TFIIIC2 subunits. In every case, it was found that the transcripts were more abundant in ovarian tumors when compared with the corresponding normal tissue from the same individual (Fig. 3). The degree of overexpression varied from 2-fold to as much as 7-fold, depending on the transcript. The same cDNAs displayed constant expression of glyceraldehyde phosphate dehydrogenase (GAPDH; Fig. 3 Bottom) and ARPP P0 genes (data not shown). It is therefore apparent that mRNAs encoding each of the five TFIIIC2 subunits are specifically and consistently overexpressed in ovarian carcinomas.
Figure 3.
Ovarian tumors express elevated levels of mRNAs encoding each of the subunits of TFIIIC2. cDNAs were generated by reverse transcription of RNA from normal ovarian tissue (N, odd lanes) or ovarian tumors (T, even lanes) from patients 1–4, as indicated. These cDNAs were PCR amplified by using primers specific for TFIIIC220, TFIIIC110, TFIIIC102, TFIIIC90, TFIIIC63, and GAPDH, as indicated.
TFIIIC2 mRNA Levels Are Not Determined by Proliferative Rate.
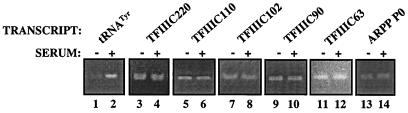
It is probable that the cancer cells analyzed here were proliferating more rapidly than the adjacent normal ovarian cells. This raises the possibility that the observed changes in the pol III transcription apparatus reflect a response to increased proliferation, rather than transformation per se. To investigate this, we carried out assays with RNA from cells that were either actively growing or had been arrested by serum deprivation. Flow cytometric analysis and measurements of thymidine incorporation into newly synthesized DNA verified that the majority of cells had withdrawn from cycle after 24 h in the absence of serum (data not shown). As expected, tRNATyr gene expression was reduced significantly in the serum-starved cells, as revealed by RT-PCR analysis of the primary transcript (Fig. 4, lanes 1 and 2). This is consistent with the fact that tRNA synthesis responds to mitogens (34). In contrast, analysis of the same cDNAs revealed little or no change in any of the mRNAs encoding the five subunits of TFIIIC2 (Fig. 4, lanes 3–14). This suggests that the genes encoding TFIIIC2 may not be regulated in response to serum levels or growth rate. It therefore seems that abnormal proliferation per se cannot account for the overexpression of TFIIIC2 in ovarian carcinomas.
Figure 4.
The rate of cell proliferation makes little difference to the levels of mRNAs encoding TFIIIC2 subunits. cDNAs were generated by reverse transcription of RNA from A31 fibroblasts that had been growth arrested by culture for 24 h in the absence of serum (odd lanes) or were actively proliferating because of continuous culture in 20% serum (even lanes). These cDNAs were PCR amplified by using primers for tRNATyr (lanes 1 and 2), TFIIIC220 (lanes 3 and 4), TFIIIC110 (lanes 5 and 6), TFIIIC102 (lanes 7 and 8), TFIIIC90 (lanes 9 and 10), TFIIIC63 (lanes 11 and 12), and ARPP P0 (lanes 13 and 14).
The DNA-Binding Activity of TFIIIC2 Is Elevated in Ovarian Tumors.
We carried out electrophoretic mobility shift assays to determine whether an increased activity of TFIIIC2 protein accompanies the elevated levels of TFIIIC2 mRNAs. By using a promoter fragment from the adenovirus VA1 gene as probe, several protein–DNA complexes were detected with extracts from normal or tumor samples (Fig. 5A). Competition experiments demonstrated that each of these complexes results from sequence-specific binding (Fig. 5A Left). Of these, the most slowly migrating complex comigrates precisely with a complex formed by TFIIIC2 that has been partially purified from HeLa cell extracts (Fig. 5A Right). Because the amount of biopsy sample available is limited, we did not investigate the identity of the high-mobility complexes; however, the region of VA1 contained in this probe has been shown to interact with additional proteins (23).
It is striking in Fig. 5A that the tumor sample generates far more of the TFIIIC2-containing complex than the sample of healthy ovarian tissue that was extracted in parallel from the same individual, patient 7. This was also true of samples prepared from other patients with ovarian cancer (Fig. 5B). In every case examined, tumor extracts gave more of the TFIIIC2-containing complex than extracts prepared in parallel from healthy ovarian tissue of the same patient. Not only was this observed in patients 1–4, who had been shown by RT-PCR to overexpress mRNAs encoding TFIIIC2 subunits; it was also found in matched samples from five additional patients (Fig. 5B and data not shown). We have therefore observed this phenomenon in nine of nine patients analyzed. Patient 7 was unusual, in that her normal tissue sample gave only very low levels of the TFIIIC2 complex (Fig. 5 A and B, lane 6). This variation between normal samples is likely to result from the heterogeneity of ovarian tissue. These experiments were repeated by using as probe an oligonucleotide containing a B-block promoter element, which is the principle binding site for TFIIIC2; again the tumor samples gave elevated levels of the TFIIIC2-containing complex (data not shown). The increased DNA-binding activity of TFIIIC2 in the tumor samples is a specific phenomenon, because none of the tumors analyzed in Fig. 5 have elevated Sp1 activity (data not shown). We conclude that deregulation of TFIIIC2 is a frequent feature of ovarian carcinomas.
Raising the Level of TFIIIC2 Can Stimulate pol III Transcription in Ovarian Epithelial Cell Extracts.
The data reveal that a collection of ovarian tumors consistently overexpress TFIIIC2 and at least four different pol III transcripts. To link these observations, we investigated whether raising the level of TFIIIC2 is sufficient to stimulate pol III transcription in extracts of ovarian cells.
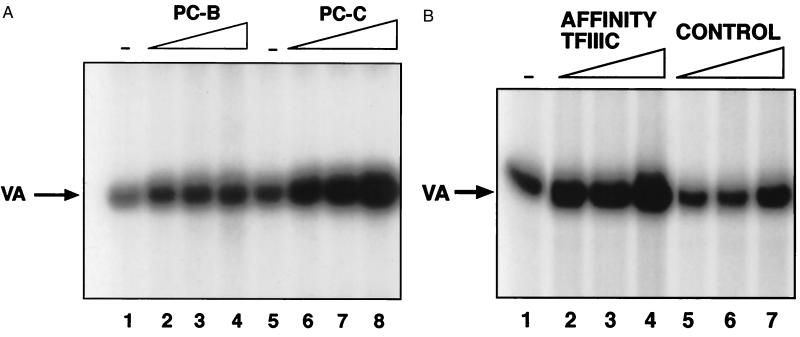
Initial experiments used crude fractions prepared by phosphocellulose chromatography according to standard procedures (30) (Fig. 6A). Pol III transcription in ovarian cell extracts was stimulated 10-fold by using a phosphocellulose C fraction that is enriched for TFIIIC2. In contrast, a phosphocellulose B fraction containing TFIIIB, but little or no TFIIIC2 produced much less effect, increasing transcription by only 2.6-fold. Further tests were carried out by using TFIIIC2 that had been affinity purified by using antiserum against its TFIIIC110 subunit. When titrated into ovarian extracts, the purified protein produced a dose-dependent increase in pol III activity (Fig. 6B). In contrast, little or no stimulation was obtained with a mock-purified fraction that had been prepared in parallel by using preimmune serum. Control experiments confirmed that the affinity-purified TFIIIC2 was free of contaminating TFIIIB or pol III (data not shown). Transcription was also stimulated in these assays by using TFIIIC2 that had been purified on a DNA affinity column (data not shown). Similar results were obtained with tRNA or 7SL genes as templates (data not shown). These data suggest that TFIIIC2 is limiting for pol III transcription in ovarian systems and that raising its level can result in elevated expression of at least some class III genes. It is therefore likely that the deregulation of TFIIIC2 found in ovarian carcinomas will have functional significance for transcriptional output.
Figure 6.
Raising the level of TFIIIC2 stimulates pol III transcription in ovarian epithelial cell extracts. (A) In vitro transcription by using 10 μg of ROSE 199 ovarian cell extract and 250 ng of pVA1 template. Reactions 2, 3, and 4 were supplemented with 2, 4, and 8 μl of phosphocellulose B, respectively. Reactions 6, 7, and 8 were supplemented with 2, 4, and 8 μl of phosphocellulose C, respectively. (B) In vitro transcription assay by using 10 μg of ROSE 199 ovarian cell extract and 250 ng of pVA1 template. Reactions 2, 3, and 4 were supplemented with 3, 6, and 9 μl, respectively, of TFIIIC that had been immunopurified by using antiserum 4286 against TFIIIC110. Reactions 5, 6, and 7 were supplemented with 3, 6, and 9 μl, respectively, of material that had been mock immunopurified by using preimmune serum.
Discussion
Ovarian cancer is the leading cause of death from gynecological malignancies in the United States (27). However, relatively little is known about the molecular mechanisms responsible for this aggressive tumor type, and the steps involved in progression from normal ovarian epithelium to invasive carcinoma are poorly understood (27). Two major reasons for this are the inaccessible position of the ovary and the fact that tumors there are frequently well advanced at the time of detection. We have identified a molecular abnormality in cases of ovarian cancer, the overexpression of TFIIIC2. Although observed previously in SV40-transformed murine cell lines (25), this phenomenon has never been reported in a human malignancy. The up-regulation of TFIIIC2 was found in each of the tumors analyzed and may therefore be widespread and, perhaps, fundamental to this disease.
Each of the tumor samples expressed abnormally high levels of tRNAArg, tRNATyr, 5S rRNA, and 7SL RNA. In the case of the tRNAs, the RT-PCR assays were specifically detecting unprocessed primary transcripts rather than the mature stable products; because of the rapid processing of these RNAs, this can be taken as an indication that pol III transcription is elevated in these cancers. This contention is supported strongly by our finding that a key pol III transcription factor is deregulated. Our observations are also consistent with a previous report that an ovarian carcinoma overexpressed BC200 RNA, a pol III transcript of unknown function; however, only one ovarian sample was examined in that study, and the mechanism responsible for deregulation was not investigated (8).
Activation of TFIIIC2 was detected in nine ovarian carcinomas that were examined. The abnormally high DNA-binding activity of TFIIIC2 in these tumors may be explained by an increase in the abundance of this factor, because RT-PCR analysis revealed a consistent overproduction of transcripts encoding each of the five subunits. However, the possibility remains that additional mechanisms may also contribute to activation, such as phosphorylation. For most pol III templates, TFIIIC2 is responsible for the initial event of promoter recognition; it then recruits TFIIIB by protein–protein interactions (1, 19). TFIIIC2 has histone acetyltransferase activity and can access promoters in a nucleosomal environment (33). Elevated levels of TFIIIC2 can therefore be expected to increase promoter occupancy in vivo. Nevertheless, it cannot be assumed that raising TFIIIC2 levels will inevitably result in higher transcriptional output, because other factors may be limiting. For example, adding more TFIIIC2 to extracts of differentiated F9 cells does not increase transcription because of a relative deficiency of TFIIIB (21). We consequently have tested the effect of raising the TFIIIC2 concentration in ovarian cell extracts and have shown that this does indeed stimulate expression of the class III genes examined. It therefore seems likely that the elevated abundance of TFIIIC2 observed in ovarian carcinomas will have a significant impact on pol III transcription.
The present study provides, to our knowledge, the first analysis of molecular events that contribute to the deregulation of pol III transcription in real tumors. Activation of TFIIIC2 does, however, have precedent in a model system of cell transformation in culture. Transformation of a fibroblast line by SV40 is accompanied by a significant increase in TFIIIC2 activity (4, 25). Adenoviral infection of HeLa cells can also activate TFIIIC2, an effect that depends on the oncoprotein E1A (20, 22–24). E1A was found to induce a specific increase in the abundance of the TFIIIC110 subunit, whereas the TFIIIC220 subunit was not affected (24). In contrast, all five of the TFIIIC2 subunit genes are overexpressed in ovarian tumors. This increase appears not to be a simple response to rapid cell proliferation within the carcinomas, because there was little or no difference in the levels of TFIIIC2 mRNAs when growth-arrested and actively cycling cells were compared. The deregulation of TFIIIC2 genes may therefore be a more specific feature of tumorigenesis rather than a growth response.
The molecular basis of TFIIIC2 overexpression in ovarian carcinomas invites investigation. A variety of potential mechanisms can be envisaged. One possibility is that the TFIIIC2 genes are amplified in tumors. Alternatively, their transcription may be targeted by factors such as E2F or Myc that themselves become activated in transformed cells. A third possibility might involve changes in the chromatin structure of TFIIIC2 genes. We can also not exclude posttranscriptional regulation, that might increase the stability of TFIIIC2 mRNAs. It will be especially interesting to examine whether a common mechanism is used to deregulate the five subunit genes, or whether the same end is achieved by distinct means in different cases. It is striking that all of the TFIIIC2 genes are consistently activated together in these carcinomas; this suggests that a shared mechanism may operate to allow coregulation. It also makes extremely unlikely the possibility that these genes become activated randomly. Serial analysis of gene expression (SAGE) showed that only 1.5% of transcripts are significantly deregulated in colorectal carcinomas, compared with normal colorectal epithelium (26). Of the 108 genes that were found by SAGE to be most substantially overexpressed in primary colorectal cancers, 48 encode ribosomal proteins, and 5 encode translation elongation factors (26). The same study observed a similar phenomenon in pancreatic carcinomas. Many other instances have also been reported in which the translation machinery has been targeted for activation in transformed cells (35, 36). By allowing elevated production of tRNA and 5S rRNA, the overexpression of TFIIIC2 would appear to provide another manifestation of a selection for changes in the protein synthetic machinery during tumor development.
Acknowledgments
This work was funded by grant 98–46 to R.J.W. from the Association for International Cancer Research and by a European Molecular Biology Organization Short Term Fellowship to allow G.S. to work in Glasgow. P.H.S. is a Wellcome Trust Career Development Fellow, and R.J.W. is a Jenner Research Fellow of the Lister Institute of Preventive Medicine.
Abbreviations
- RT-PCR
reverse transcription–PCR
- EMSA
electrophoretic mobility shift assay
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230224097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230224097
References
- 1.White R J. RNA Polymerase III Transcription. Berlin: Springer; 1998. [Google Scholar]
- 2.Scott M R D, Westphal K-H, Rigby P W J. Cell. 1983;34:557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- 3.Singh K, Carey M, Saragosti S, Botchan M. Nature (London) 1985;314:553–556. doi: 10.1038/314553a0. [DOI] [PubMed] [Google Scholar]
- 4.White R J, Stott D, Rigby P W J. EMBO J. 1990;9:3713–3721. doi: 10.1002/j.1460-2075.1990.tb07584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottesfeld J M, Johnson D L, Nyborg J K. Mol Cell Biol. 1996;16:1777–1785. doi: 10.1128/mcb.16.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H-D, Trivedi A, Johnson D L. Mol Cell Biol. 1997;17:6838–6846. doi: 10.1128/mcb.17.12.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Heierhorst J, Brosius J, Tiedge H. Eur J Cancer. 1997;33:288–292. doi: 10.1016/s0959-8049(96)00453-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Bocker W, Brosius J, Tiedge H. J Pathol. 1997;183:345–351. doi: 10.1002/(SICI)1096-9896(199711)183:3<345::AID-PATH930>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Baxter G C, Stanners C P. J Cell Physiol. 1978;96:139–146. doi: 10.1002/jcp.1040960202. [DOI] [PubMed] [Google Scholar]
- 10.Brooks R F. Cell. 1977;12:311–317. doi: 10.1016/0092-8674(77)90209-4. [DOI] [PubMed] [Google Scholar]
- 11.Ronning O W, Lindmo T, Pettersen E O, Seglen P O. J Cell Physiol. 1981;109:411–418. doi: 10.1002/jcp.1041090306. [DOI] [PubMed] [Google Scholar]
- 12.White R J, Trouche D, Martin K, Jackson S P, Kouzarides T. Nature (London) 1996;382:88–90. doi: 10.1038/382088a0. [DOI] [PubMed] [Google Scholar]
- 13.Larminie C G C, Cairns C A, Mital R, Martin K, Kouzarides T, Jackson S P, White R J. EMBO J. 1997;16:2061–2071. doi: 10.1093/emboj/16.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu W-M, Wang Z, Roeder R G, Schmid C W. J Biol Chem. 1997;272:14755–14761. doi: 10.1074/jbc.272.23.14755. [DOI] [PubMed] [Google Scholar]
- 15.Mulligan G, Jacks T. Trends Genet. 1998;14:223–229. doi: 10.1016/s0168-9525(98)01470-x. [DOI] [PubMed] [Google Scholar]
- 16.Chesnokov I, Chu W-M, Botchan M R, Schmid C W. Mol Cell Biol. 1996;16:7084–7088. doi: 10.1128/mcb.16.12.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cairns C A, White R J. EMBO J. 1998;17:3112–3123. doi: 10.1093/emboj/17.11.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollstein M, Rice K, Greenblatt M S, Soussi T, Fucks R, Sorlie T, Hovig E, Smith-Sorensen B, Montesano R, Harris C C. Nucleic Acids Res. 1994;22:3551–3555. [PMC free article] [PubMed] [Google Scholar]
- 19.Paule M R, White R J. Nucleic Acids Res. 2000;28:1283–1298. doi: 10.1093/nar/28.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoeffler W K, Roeder R G. Cell. 1985;41:955–963. doi: 10.1016/s0092-8674(85)80076-3. [DOI] [PubMed] [Google Scholar]
- 21.White R J, Stott D, Rigby P W J. Cell. 1989;59:1081–1092. doi: 10.1016/0092-8674(89)90764-2. [DOI] [PubMed] [Google Scholar]
- 22.Yoshinaga S, Dean N, Han M, Berk A J. EMBO J. 1986;5:343–354. doi: 10.1002/j.1460-2075.1986.tb04218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoeffler W K, Kovelman R, Roeder R G. Cell. 1988;53:907–920. doi: 10.1016/s0092-8674(88)90409-6. [DOI] [PubMed] [Google Scholar]
- 24.Sinn E, Wang Z, Kovelman R, Roeder R G. Genes Dev. 1995;9:675–685. doi: 10.1101/gad.9.6.675. [DOI] [PubMed] [Google Scholar]
- 25.Larminie C G C, Sutcliffe J E, Tosh K, Winter A G, Felton-Edkins Z A, White R J. Mol Cell Biol. 1999;19:4927–4934. doi: 10.1128/mcb.19.7.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Zhou W, Velculescu V E, Kern S E, Hruban R H, Hamilton S R, Vogelstein B, Kinzler K W. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 27.Gallion H H, Pieretti M, DePriest P D, van Nagell J R., Jr Cancer. 1995;76:1992–1997. doi: 10.1002/1097-0142(19951115)76:10+<1992::aid-cncr2820761315>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 28.White R J, Gottlieb T M, Downes C S, Jackson S P. Mol Cell Biol. 1995;15:1983–1992. doi: 10.1128/mcb.15.4.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams A T, Auersperg N. Exp Cell Biol. 1985;53:181–188. doi: 10.1159/000163311. [DOI] [PubMed] [Google Scholar]
- 30.Segall J, Matsui T, Roeder R G. J Biol Chem. 1980;255:11986–11991. [PubMed] [Google Scholar]
- 31.Cormack B P, Struhl K. Cell. 1992;69:685–696. doi: 10.1016/0092-8674(92)90232-2. [DOI] [PubMed] [Google Scholar]
- 32.Yoshinaga S K, L'Etoile N D, Berk A J. J Biol Chem. 1989;264:10726–10731. [PubMed] [Google Scholar]
- 33.Kundu T K, Wang Z, Roeder R G. Mol Cell Biol. 1999;19:1605–1615. doi: 10.1128/mcb.19.2.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson L F, Abelson H T, Green H, Penman S. Cell. 1974;1:95–100. [Google Scholar]
- 35.Sonenberg N. Curr Opin Cell Biol. 1993;5:955–960. doi: 10.1016/0955-0674(93)90076-3. [DOI] [PubMed] [Google Scholar]
- 36.Rosenwald I B. BioEssays. 1996;18:243–250. doi: 10.1002/bies.950180312. [DOI] [PubMed] [Google Scholar]