Abstract
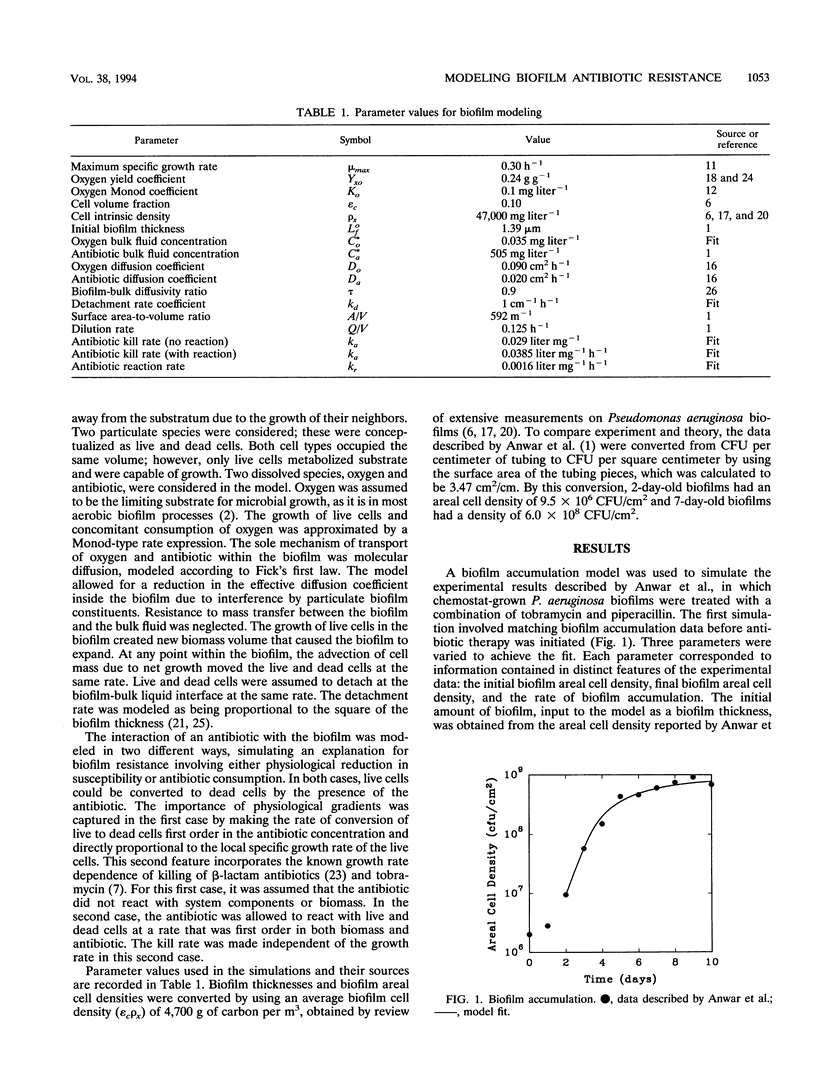
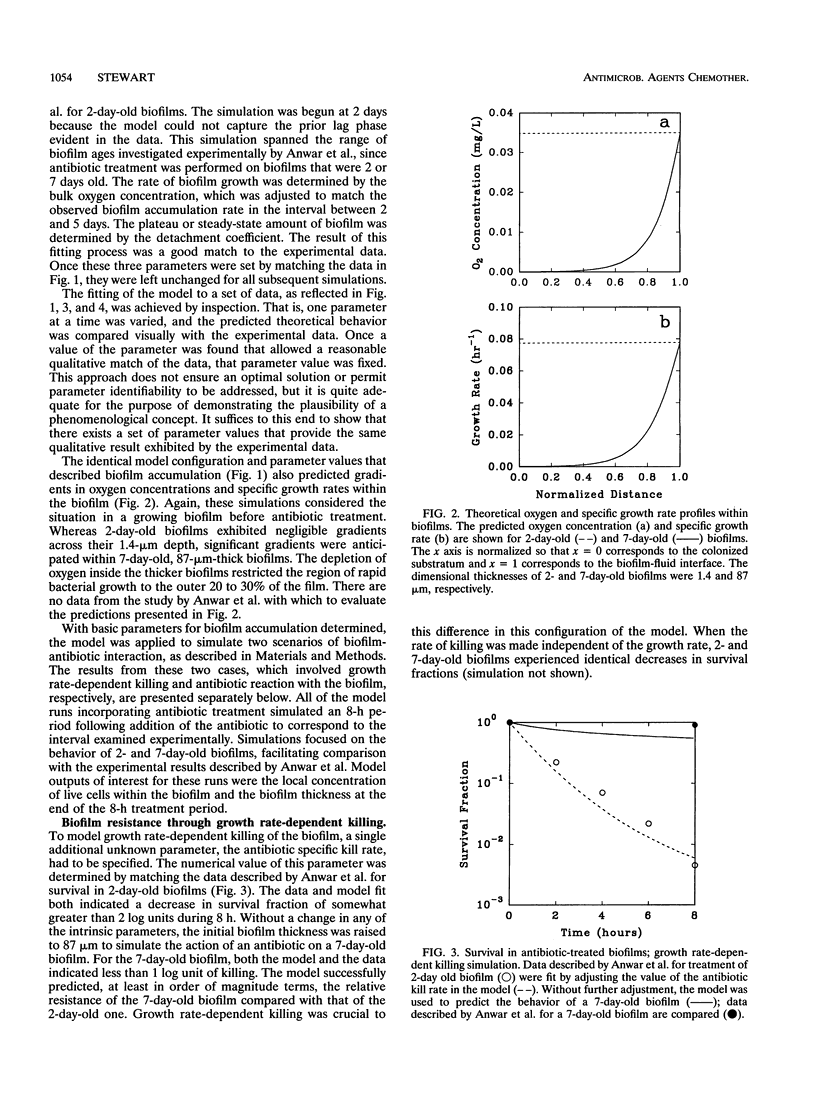
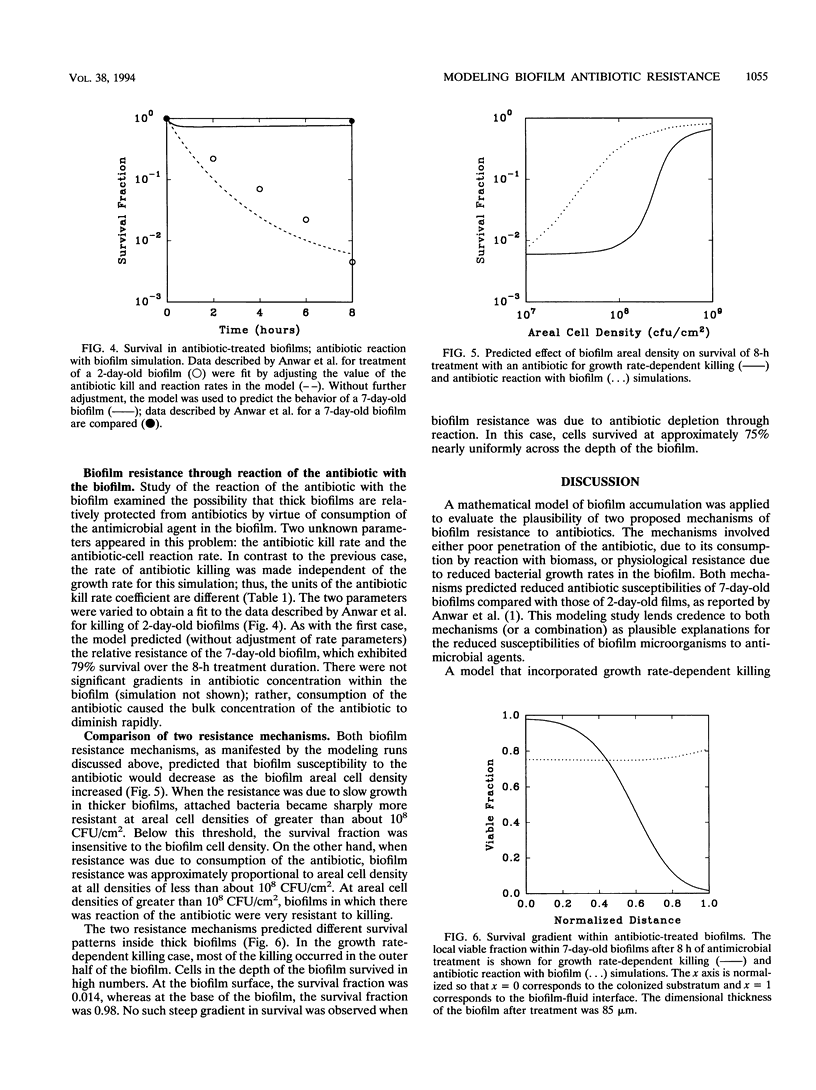
A computer model of biofilm dynamics was adapted to incorporate the activity of an antimicrobial agent on bacterial biofilm. The model was used to evaluate the plausibility of two mechanisms of biofilm antibiotic resistance by qualitative comparison with data from a well-characterized experimental system (H. Anwar, J. L. Strap, and J. W. Costerton, Antimicrob. Agents Chemother. 36:1208-1214, 1992). The two mechanisms involved either depletion of the antibiotic by reaction with biomass or physiological resistance due to reduced bacterial growth rates in the biofilm. Both mechanisms predicted the experimentally observed resistance of 7-day-old Pseudomonas aeruginosa biofilms compared with that of 2-day-old ones. A version of the model that incorporated growth rate-dependent killing predicted reduced susceptibility of thicker biofilms because oxygen was exhausted within these biofilms, leading to very slow growth in part of the biofilm. A version of the model that incorporated a destructive reaction of the antibiotic with biomass likewise accounted for the relative resistance of thicker biofilms. Resistance in this latter case was due to depletion of the antibiotic in the bulk fluid rather than development of a gradient in the antibiotic concentration within the biofilm. The modeling results predicted differences between the two cases, such as in the survival profiles within the biofilm, that could permit these resistance mechanisms to be experimentally distinguished.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar H., Strap J. L., Chen K., Costerton J. W. Dynamic interactions of biofilms of mucoid Pseudomonas aeruginosa with tobramycin and piperacillin. Antimicrob Agents Chemother. 1992 Jun;36(6):1208–1214. doi: 10.1128/aac.36.6.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Allison D. G., Gilbert P. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother. 1988 Dec;22(6):777–780. doi: 10.1093/jac/22.6.777. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Dibdin G. H. A finite-difference computer model of solute diffusion in bacterial films with simultaneous metabolism and chemical reaction. Comput Appl Biosci. 1992 Oct;8(5):489–500. doi: 10.1093/bioinformatics/8.5.489. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Brown M. R., Allison D. G., Gilbert P. Susceptibility of bacterial biofilms to tobramycin: role of specific growth rate and phase in the division cycle. J Antimicrob Chemother. 1990 Apr;25(4):585–591. doi: 10.1093/jac/25.4.585. [DOI] [PubMed] [Google Scholar]
- Gilbert P., Collier P. J., Brown M. R. Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent response. Antimicrob Agents Chemother. 1990 Oct;34(10):1865–1868. doi: 10.1128/aac.34.10.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle B. D., Costerton J. W. Bacterial resistance to antibiotics: the role of biofilms. Prog Drug Res. 1991;37:91–105. doi: 10.1007/978-3-0348-7139-6_2. [DOI] [PubMed] [Google Scholar]
- Leitão J. H., Fialho A. M., Sá-Correia I. Effects of growth temperature on alginate synthesis and enzymes in Pseudomonas aeruginosa variants. J Gen Microbiol. 1992 Mar;138(3):605–610. doi: 10.1099/00221287-138-3-605. [DOI] [PubMed] [Google Scholar]
- Nichols W. W., Evans M. J., Slack M. P., Walmsley H. L. The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol. 1989 May;135(5):1291–1303. doi: 10.1099/00221287-135-5-1291. [DOI] [PubMed] [Google Scholar]
- Rodriguez G. G., Phipps D., Ishiguro K., Ridgway H. F. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol. 1992 Jun;58(6):1801–1808. doi: 10.1128/aem.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Cozens R., Tosch W., Zak O., Tomasz A. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol. 1986 May;132(5):1297–1304. doi: 10.1099/00221287-132-5-1297. [DOI] [PubMed] [Google Scholar]



