Abstract
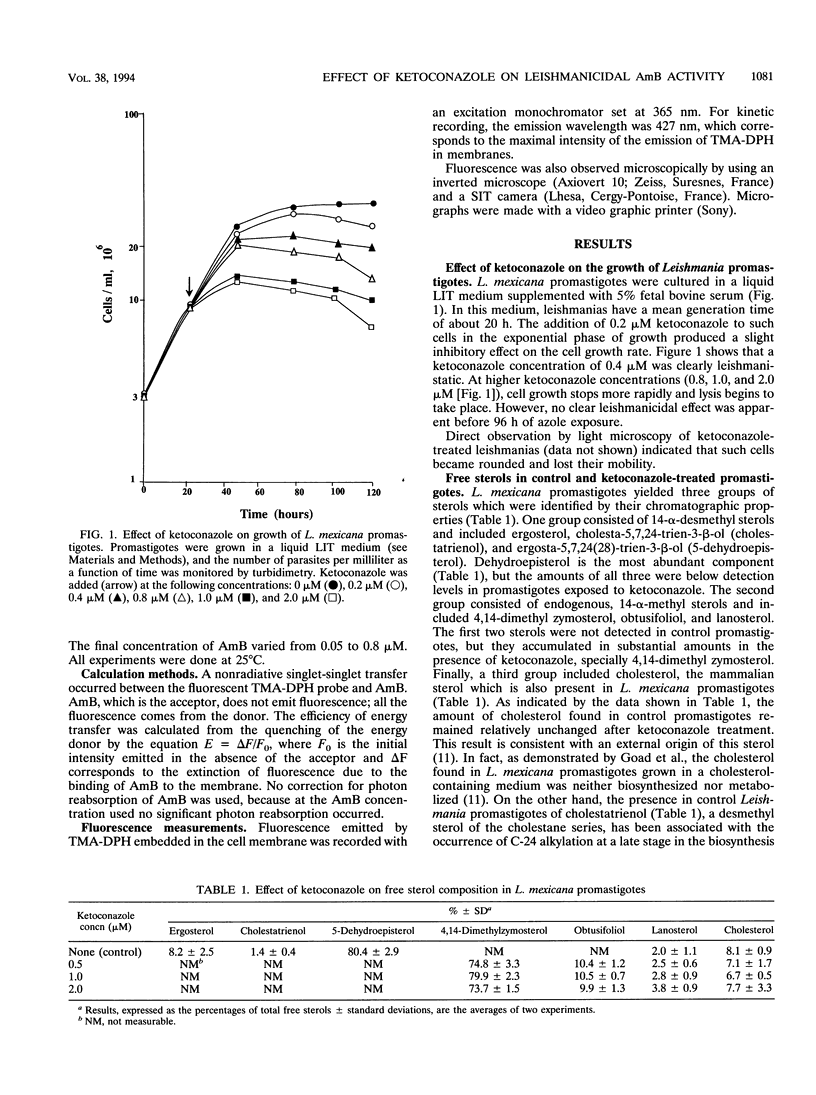
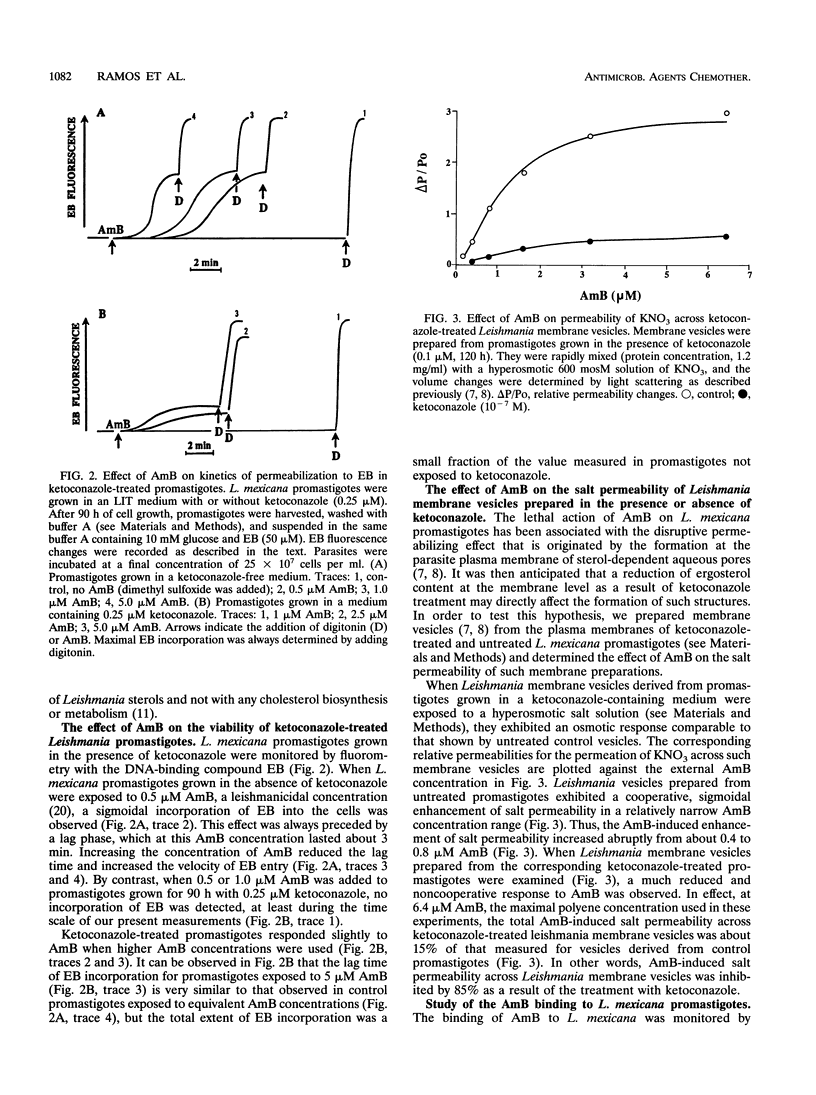
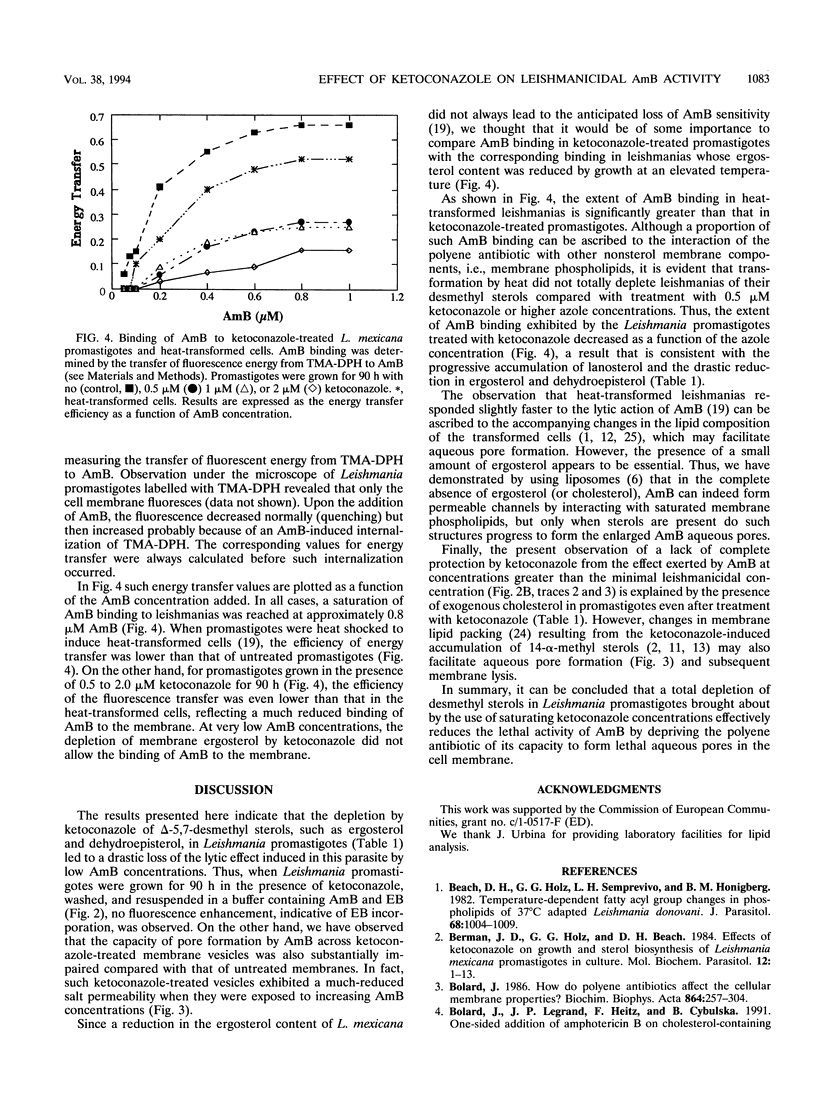
The effect of ergosterol depletion by ketoconazole on the leishmanicidal activity of the pore-forming antibiotic amphotericin B (AmB) was investigated. Leishmania mexicana promastigotes were lysed within minutes by the addition of micromolar concentrations of AmB (0.5 microM) but became insensitive to AmB after growth in the presence of ketoconazole (0.25 microM, 90 h). Lipid chromatographic analysis indicated that under such conditions, ketoconazole depleted the major Leishmania sterols, dehydroepisterol and ergosterol. Plasma membrane vesicles prepared from ketoconazole-treated promastigotes exhibited a much reduced enhancement of their salt permeability after the addition of AmB at concentrations as high as 5 microM. This finding clearly indicates that upon ketoconazole treatment, the capacity of pore formation by the antibiotic is substantially impaired. The reduction of desmethyl sterols by ketoconazole was accompanied by a significant increase of 14-alpha-methyl sterols, but exogenous cholesterol remained unchanged. This ability of Leishmania promastigotes to incorporate cholesterol from the external medium may explain why ketoconazole-treated cells exhibited a much decreased but significative response to AmB when they were exposed to high AmB concentrations (2.5 or 5.0 microM). Parallel measurements by using a fluorescence energy transfer method indicated that binding of AmB to ketoconazole-treated Leishmania promastigotes and heat-transformed leishmanias was also decreased but to different extents, a finding that may be related to the differences in their sterol content. The results obtained clearly indicate that the specific interaction of AmB with desmethyl sterols, such as dehydroepisterol, ergosterol, and even exogenous cholesterol, is an absolute requirement for the lethal action exerted by this polyene antibiotic on L. mexicana promastigotes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach D. H., Holz G. G., Jr, Semprevivo L. H., Honigberg B. M. Temperature-dependent fatty acyl group changes in phospholipids of 37 C-adapted Leishmania donovani promastigotes. J Parasitol. 1982 Dec;68(6):1004–1009. [PubMed] [Google Scholar]
- Berman J. D., Holz G. G., Jr, Beach D. H. Effects of ketoconazole on growth and sterol biosynthesis of Leishmania mexicana promastigotes in culture. Mol Biochem Parasitol. 1984 May;12(1):1–13. doi: 10.1016/0166-6851(84)90039-2. [DOI] [PubMed] [Google Scholar]
- Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986 Dec 22;864(3-4):257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Brajtburg J., Powderly W. G., Kobayashi G. S., Medoff G. Amphotericin B: current understanding of mechanisms of action. Antimicrob Agents Chemother. 1990 Feb;34(2):183–188. doi: 10.1128/aac.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B. E. A sequential mechanism for the formation of aqueous channels by amphotericin B in liposomes. The effect of sterols and phospholipid composition. Biochim Biophys Acta. 1992 Jul 8;1108(1):49–58. doi: 10.1016/0005-2736(92)90113-z. [DOI] [PubMed] [Google Scholar]
- Cohen B. E., Gamargo M. Concentration and time dependence of amphotericin B-induced permeability changes across plasma membrane vesicles from Leishmania sp. Drugs Exp Clin Res. 1987;13(9):539–546. [PubMed] [Google Scholar]
- Cohen B. E., Ramos H., Gamargo M., Urbina J. The water and ionic permeability induced by polyene antibiotics across plasma membrane vesicles from Leishmania sp. Biochim Biophys Acta. 1986 Aug 7;860(1):57–65. doi: 10.1016/0005-2736(86)90498-0. [DOI] [PubMed] [Google Scholar]
- Croft S. L. Recent developments in the chemotherapy of leishmaniasis. Trends Pharmacol Sci. 1988 Oct;9(10):376–381. doi: 10.1016/0165-6147(88)90258-1. [DOI] [PubMed] [Google Scholar]
- Davidson R. N., Croft S. L., Scott A., Maini M., Moody A. H., Bryceson A. D. Liposomal amphotericin B in drug-resistant visceral leishmaniasis. Lancet. 1991 May 4;337(8749):1061–1062. doi: 10.1016/0140-6736(91)91708-3. [DOI] [PubMed] [Google Scholar]
- Goad L. J., Holz G. G., Jr, Beach D. H. Sterols of ketoconazole-inhibited Leishmania mexicana mexicana promastigotes. Mol Biochem Parasitol. 1985 Jun;15(3):257–279. doi: 10.1016/0166-6851(85)90089-1. [DOI] [PubMed] [Google Scholar]
- Hart D. T., Lauwers W. J., Willemsens G., Vanden Bossche H., Opperdoes F. R. Perturbation of sterol biosynthesis by itraconazole and ketoconazole in Leishmania mexicana mexicana infected macrophages. Mol Biochem Parasitol. 1989 Mar 1;33(2):123–134. doi: 10.1016/0166-6851(89)90026-1. [DOI] [PubMed] [Google Scholar]
- Henry-Toulmé N., Seman M., Bolard J. Interaction of amphotericin B and its N-fructosyl derivative with murine thymocytes: a comparative study using fluorescent membrane probes. Biochim Biophys Acta. 1989 Jul 10;982(2):245–252. doi: 10.1016/0005-2736(89)90061-8. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Feingold D. S. Polyene antibiotic action on lecithin liposomes: effect of cholesterol and fatty acyl chains. Biochem Biophys Res Commun. 1973 Apr 16;51(4):972–978. doi: 10.1016/0006-291x(73)90022-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ramos H., Milhaud J., Cohen B. E., Bolard J. Enhanced action of amphotericin B on Leishmania mexicana resulting from heat transformation. Antimicrob Agents Chemother. 1990 Aug;34(8):1584–1589. doi: 10.1128/aac.34.8.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A. K., Mukherjee T., Bhaduri A. Mechanism of action of amphotericin B on Leishmania donovani promastigotes. Mol Biochem Parasitol. 1986 Jun;19(3):195–200. doi: 10.1016/0166-6851(86)90001-0. [DOI] [PubMed] [Google Scholar]
- Sokol-Anderson M., Sligh J. E., Jr, Elberg S., Brajtburg J., Kobayashi G. S., Medoff G. Role of cell defense against oxidative damage in the resistance of Candida albicans to the killing effect of amphotericin B. Antimicrob Agents Chemother. 1988 May;32(5):702–705. doi: 10.1128/aac.32.5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Effect of ketoconazole on the fungicidal action of amphotericin B in Candida albicans. Antimicrob Agents Chemother. 1983 Jan;23(1):185–187. doi: 10.1128/aac.23.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina J. A., Vivas J., Ramos H., Larralde G., Aguilar Z., Avilán L. Alteration of lipid order profile and permeability of plasma membranes from Trypanosoma cruzi epimastigotes grown in the presence of ketoconazole. Mol Biochem Parasitol. 1988 Aug;30(2):185–195. doi: 10.1016/0166-6851(88)90111-9. [DOI] [PubMed] [Google Scholar]



