Abstract
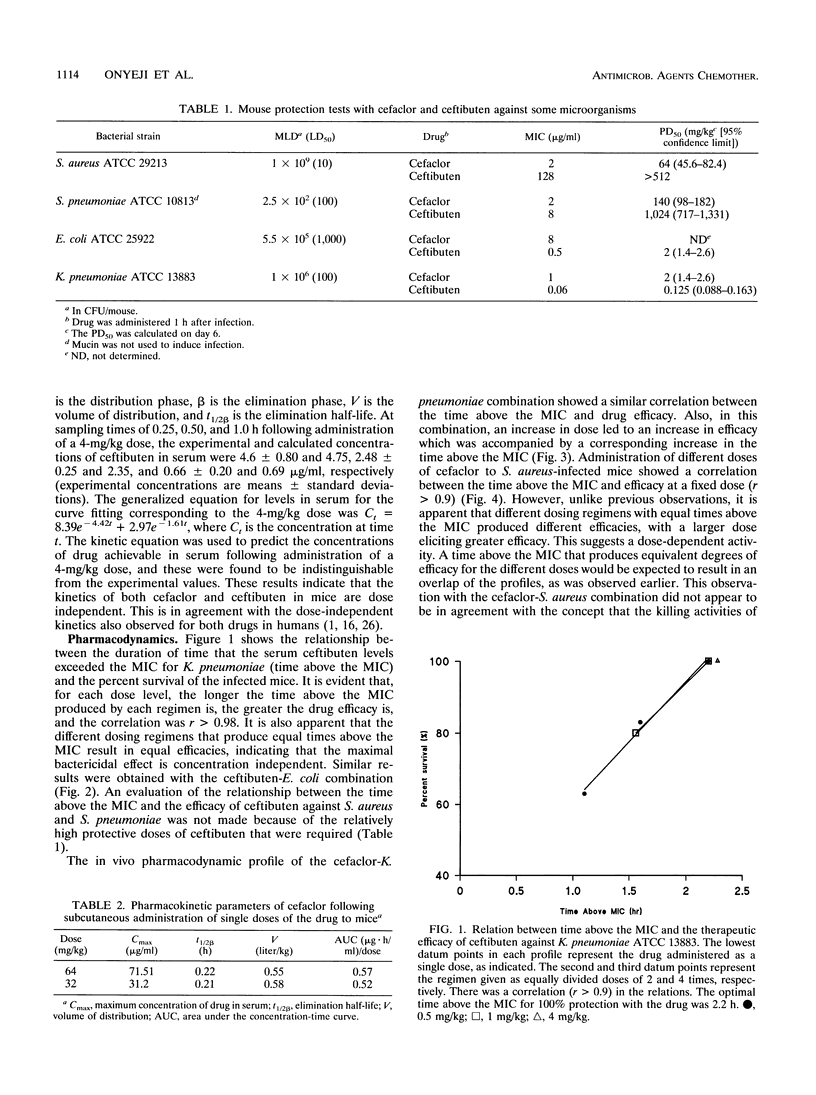
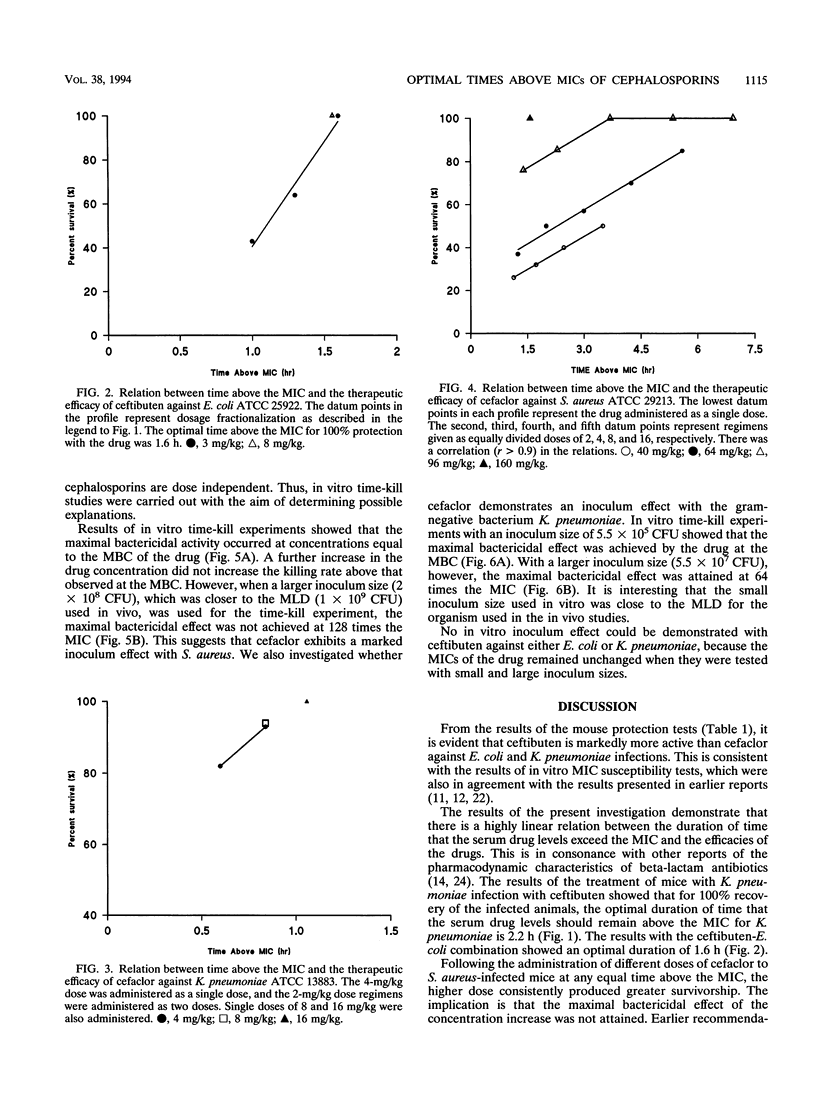
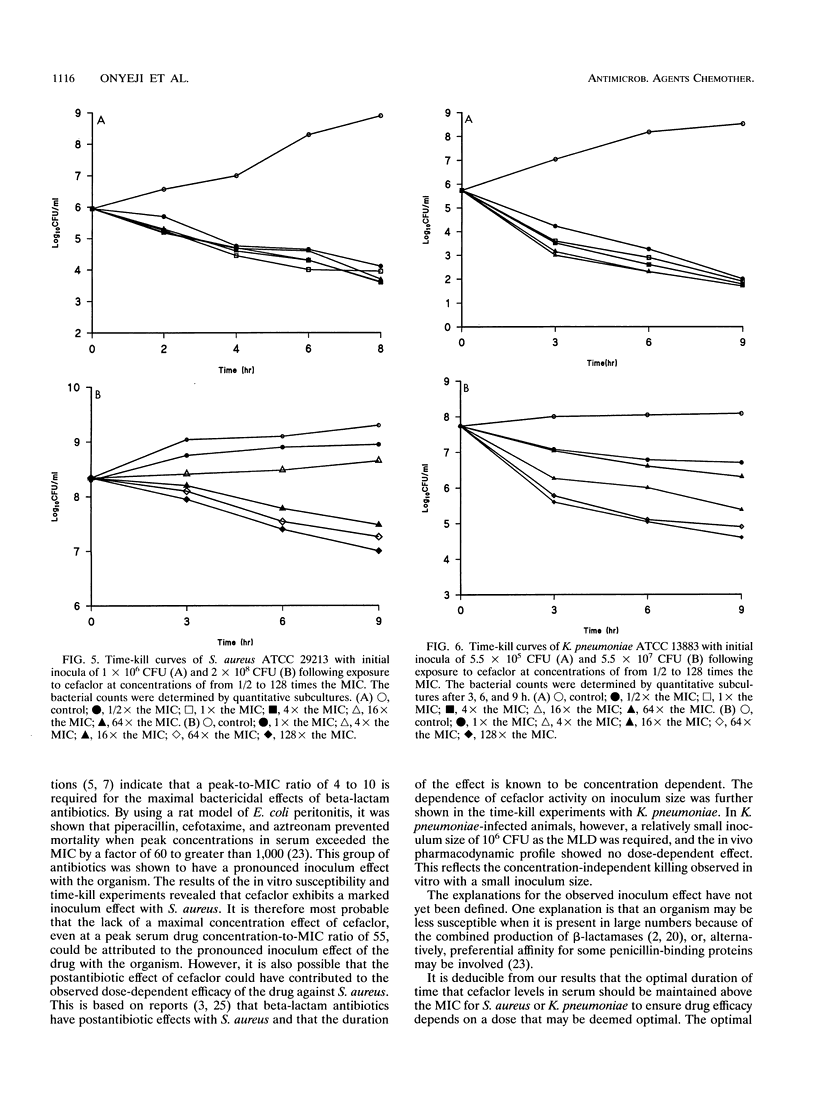
The duration of time that serum drug levels remain above the MIC (time above the MIC) for the pathogen has been shown to be the most significant parameter determining the efficacies of beta-lactam antibiotics. In the described study, we investigated the optimal time above the MIC of ceftibuten and cefaclor using a nonneutropenic mouse model of intra-abdominal infections caused by Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, and Streptococcus pneumoniae. The abilities of the drugs to protect mice against the organisms were determined in mouse protection tests, and the doses were fractionated to produce various dosing regimens with different times above the MIC. All drug-organism combinations showed a significant correlation (r > 0.9) between drug efficacy and the time above the MIC. Also, with ceftibuten treatment, the different dosing regimens that produced equal times above the MIC resulted in the same efficacy, whereas with cefaclor, an apparent dose-dependent effect was observed. These results showed that for a 100% recovery from K. pneumoniae and E. coli infections, the optimal times above the MIC with ceftibuten treatment were 2.2 and 1.6 h, respectively. Relatively high doses of both antibiotics were required to ensure recovery from S. pneumoniae infections. In vitro time-kill studies demonstrated that cefaclor exhibits a marked inoculum effect against the pathogens, and there was a concentration-dependent killing at a large inoculum size. On the other hand, ceftibuten showed no inoculum effect. It is suggested that optimization of both dose and time above the MIC appears to be necessary for the treatment of S. aureus infections with cefaclor, and this may apply to other beta-lactams tht exhibit marked inoculum effects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbhaiya R. H., Shukla U. A., Gleason C. R., Shyu W. C., Wilber R. B., Pittman K. A. Comparison of cefprozil and cefaclor pharmacokinetics and tissue penetration. Antimicrob Agents Chemother. 1990 Jun;34(6):1204–1209. doi: 10.1128/aac.34.6.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger R. R., Washington J. A., 2nd Effect of inoculum size and beta-lactamase production on in vitro activity of new cephalosporins against Haemophilus species. Antimicrob Agents Chemother. 1980 Mar;17(3):393–396. doi: 10.1128/aac.17.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundtzen R. W., Gerber A. U., Cohn D. L., Craig W. A. Postantibiotic suppression of bacterial growth. Rev Infect Dis. 1981 Jan-Feb;3(1):28–37. doi: 10.1093/clinids/3.1.28. [DOI] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Comparative antibacterial activity of a new oral cephalosporin, BMY-28100. Antimicrob Agents Chemother. 1987 Mar;31(3):480–483. doi: 10.1128/aac.31.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. A., Ebert S. C. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis Suppl. 1990;74:63–70. [PubMed] [Google Scholar]
- Drusano G. L. Human pharmacodynamics of beta-lactams, aminoglycosides and their combination. Scand J Infect Dis Suppl. 1990;74:235–248. [PubMed] [Google Scholar]
- Ellner P. D., Neu H. C. The inhibitory quotient. A method for interpreting minimum inhibitory concentration data. JAMA. 1981 Oct 2;246(14):1575–1578. doi: 10.1001/jama.246.14.1575. [DOI] [PubMed] [Google Scholar]
- Gerber A. U., Brugger H. P., Feller C., Stritzko T., Stalder B. Antibiotic therapy of infections due to Pseudomonas aeruginosa in normal and granulocytopenic mice: comparison of murine and human pharmacokinetics. J Infect Dis. 1986 Jan;153(1):90–97. doi: 10.1093/infdis/153.1.90. [DOI] [PubMed] [Google Scholar]
- Gerber A. U. Impact of the antibiotic dosage schedule on efficacy in experimental soft tissue infections. Scand J Infect Dis Suppl. 1990;74:147–154. [PubMed] [Google Scholar]
- Gerber A. U., Stritzko T., Segessenmann C., Stalder B. Simulation of human pharmacokinetic profiles in mice, and impact on antimicrobial efficacy of netilmicin, ticarcillin and ceftazidime in the peritonitis-septicemia model. Scand J Infect Dis Suppl. 1990;74:195–203. [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Ceftibuten (7432-S, SCH 39720): comparative antimicrobial activity against 4735 clinical isolates, beta-lactamase stability and broth microdilution quality control guidelines. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):802–807. doi: 10.1007/BF01975055. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. In-vitro antimicrobial activity of 7432-S (SCH39720) against commonly isolated respiratory tract pathogens. J Antimicrob Chemother. 1988 Sep;22(3):387–389. doi: 10.1093/jac/22.3.387-a. [DOI] [PubMed] [Google Scholar]
- Kammer R. B., Short L. J. Cefaclor--summary of clinical experience. Postgrad Med J. 1979;55 (Suppl 4):93–99. [PubMed] [Google Scholar]
- Leggett J. E., Fantin B., Ebert S., Totsuka K., Vogelman B., Calame W., Mattie H., Craig W. A. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J Infect Dis. 1989 Feb;159(2):281–292. doi: 10.1093/infdis/159.2.281. [DOI] [PubMed] [Google Scholar]
- Mattie H., van Dokkum A. M., Brus-Weijer L., Krul A. M., van Strijen E. Antibacterial activity of four cephalosporins in an experimental infection in relation to in vitro effect and pharmacokinetics. J Infect Dis. 1990 Sep;162(3):717–722. doi: 10.1093/infdis/162.3.717. [DOI] [PubMed] [Google Scholar]
- Nakashima M., Uematsu T., Takiguchi Y., Mizuno A., Iida M., Yoshida T., Yamamoto S., Kitagawa K., Oguma T., Ishii H. Phase I clinical studies of 7432-S, a new oral cephalosporin: safety and pharmacokinetics. J Clin Pharmacol. 1988 Mar;28(3):246–252. doi: 10.1002/j.1552-4604.1988.tb03140.x. [DOI] [PubMed] [Google Scholar]
- Quintiliani R., Nightingale C. H., Crowe H. M., Cooper B. W., Bartlett R. C., Gousse G. Strategic antibiotic decision-making at the formulary level. Rev Infect Dis. 1991 Jul-Aug;13 (Suppl 9):S770–S777. doi: 10.1093/clinids/13.supplement_9.s770. [DOI] [PubMed] [Google Scholar]
- Roosendaal R., Bakker-Woudenberg I. A. Impact of the antibiotic dosage schedule on efficacy in experimental lung infections. Scand J Infect Dis Suppl. 1990;74:155–162. [PubMed] [Google Scholar]
- Roosendaal R., Bakker-Woudenberg I. A., van den Berg J. C., Michel M. F. Therapeutic efficacy of continuous versus intermittent administration of ceftazidime in an experimental Klebsiella pneumoniae pneumonia in rats. J Infect Dis. 1985 Aug;152(2):373–378. doi: 10.1093/infdis/152.2.373. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Garner C., Wilcox C., Finland M. Effect of inoculum and of beta-lactamase on the anti-staphylococcal activity of thirteen penicillins and cephalosporins. Antimicrob Agents Chemother. 1975 Sep;8(3):344–349. doi: 10.1128/aac.8.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawar R., LaRocco M., Cleary T. G. Comparative in vitro activity of ceftibuten (Sch 39720) against bacterial enteropathogens. Antimicrob Agents Chemother. 1989 May;33(5):781–784. doi: 10.1128/aac.33.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano F., Ponte C., Santamaría M., Jimenez-Arriero M. Relevance of the inoculum effect of antibiotics in the outcome of experimental infections caused by Escherichia coli. J Antimicrob Chemother. 1990 Apr;25(4):621–627. doi: 10.1093/jac/25.4.621. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Gudmundsson S., Leggett J., Turnidge J., Ebert S., Craig W. A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988 Oct;158(4):831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Gudmundsson S., Turnidge J., Leggett J., Craig W. A. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J Infect Dis. 1988 Feb;157(2):287–298. doi: 10.1093/infdis/157.2.287. [DOI] [PubMed] [Google Scholar]
- Wise R., Nye K., O'Neill P., Wostenholme M., Andrews J. M. Pharmacokinetics and tissue penetration of ceftibuten. Antimicrob Agents Chemother. 1990 Jun;34(6):1053–1055. doi: 10.1128/aac.34.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]



