Abstract
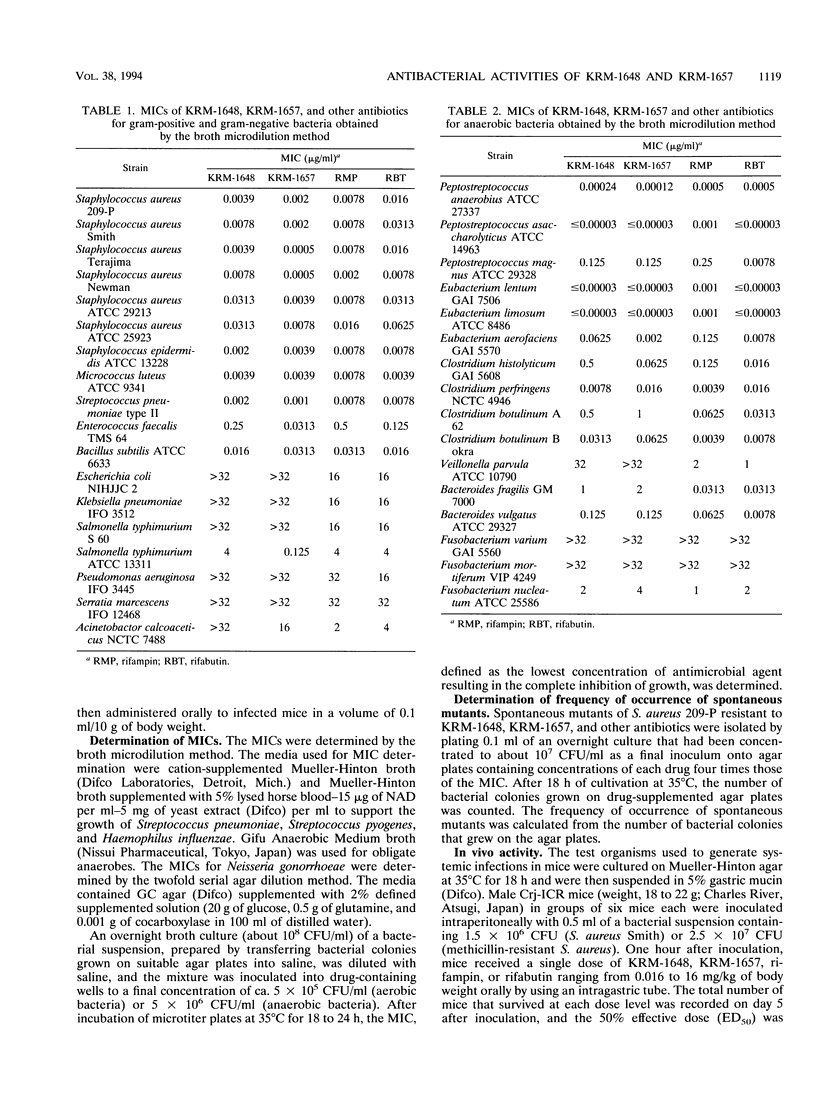
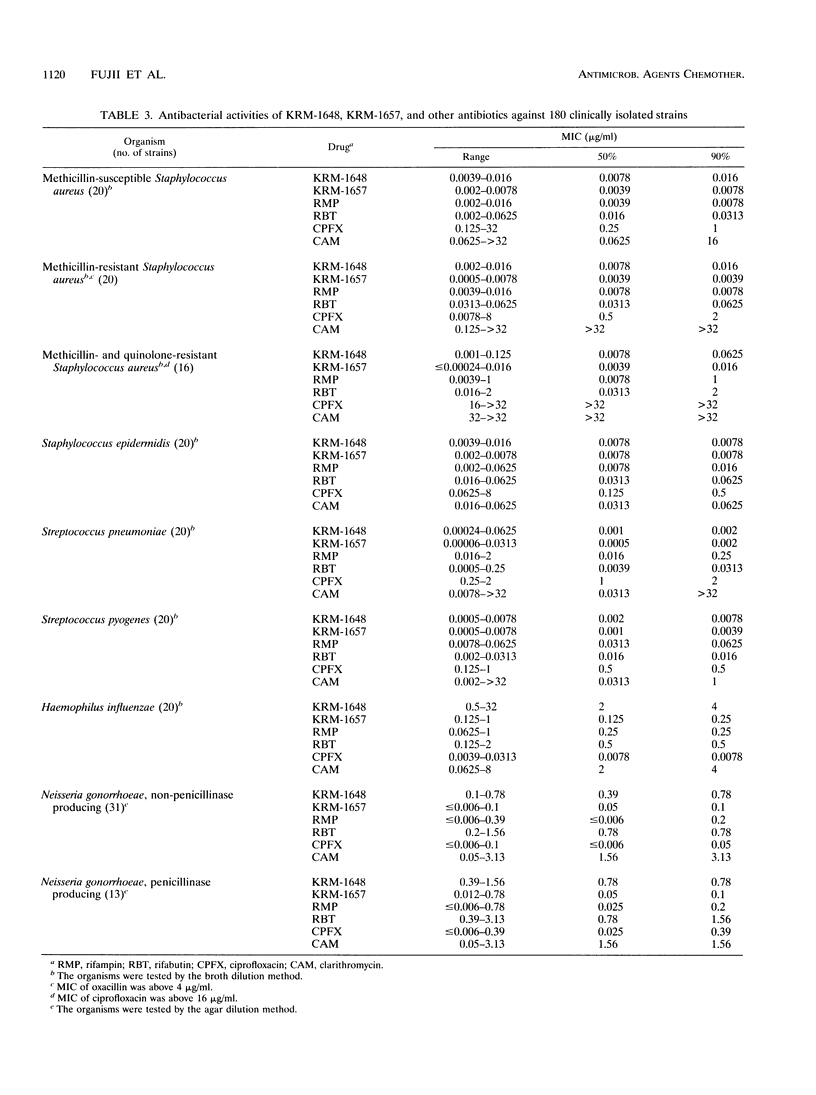
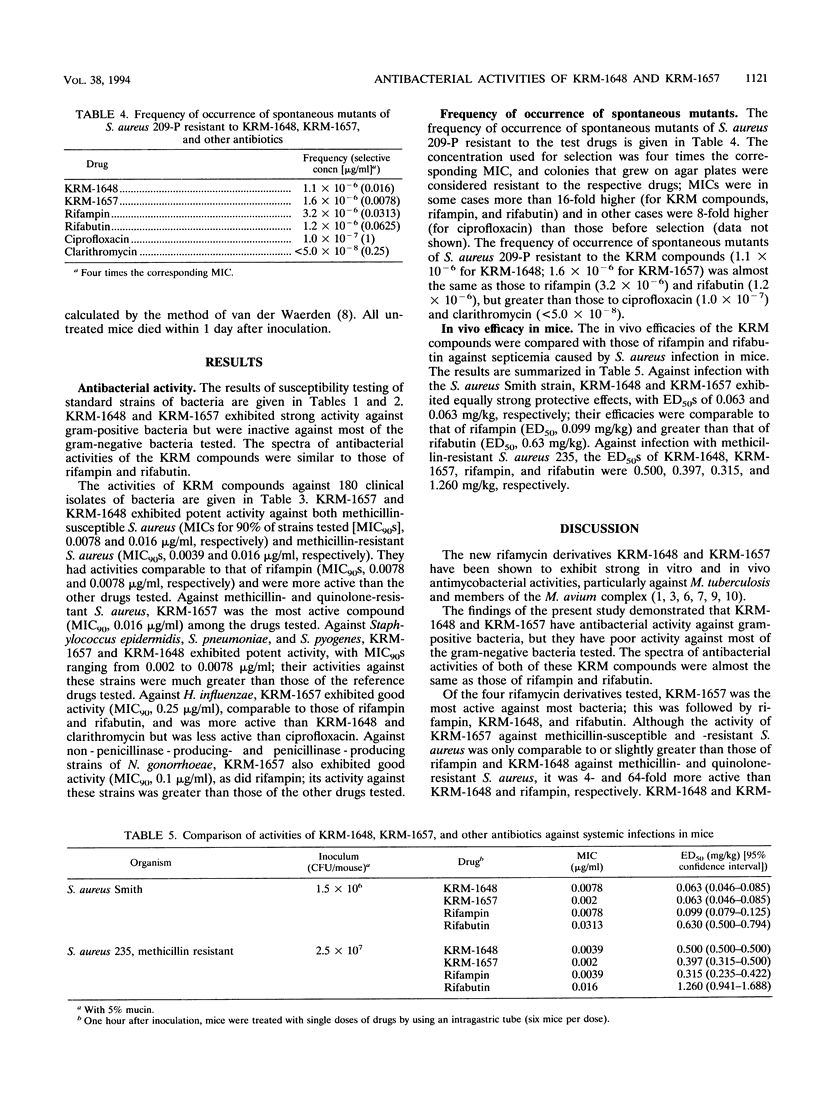
The in vitro and in vivo antibacterial activities of the new rifamycin derivatives KRM-1648 and KRM-1657 were compared with those of rifampin. Rifabutin, ciprofloxacin, and clarithromycin were also tested for reference. The respective MICs of KRM-1648 and KRM-1657 for 90% of the strains tested (MIC90S) were 0.016 and 0.0078 microgram/ml, respectively, for methicillin-susceptible Staphylococcus aureus, 0.016 and 0.0039 microgram/ml, respectively, for methicillin-resistant S. aureus, and 0.0625 and 0.016 microgram/ml, respectively, for methicillin- and quinolone-resistant S. aureus. These MIC90S of KRM-1657 were equal to or 2- to 64-fold lower than those of rifampin. KRM-1648 and KRM-1657 with MIC90S of between 0.002 and 0.078 microgram/ml were 2- to 128-fold more active than rifampin against Staphylococcus epidermidis and Streptococcus species, including Streptococcus pneumoniae and Streptococcus pyogenes. The MIC90S of KRM-1657 for Haemophilus influenzae and Neisseria gonorrhoeae were 0.25 and 0.1 microgram/ml, respectively; KRM-1657 was almost as active as rifampin and was 8- to 16-fold more active than KRM-1648 against these strains. The frequency of occurrence of spontaneous mutations to resistance to KRM-1648 and KRM-1657 was equal to that to rifampin. Against systemic infection with S. aureus in mice, the efficacies of KRM-1648 and KRM-1657 were comparable to that of rifampin.
Full text
PDF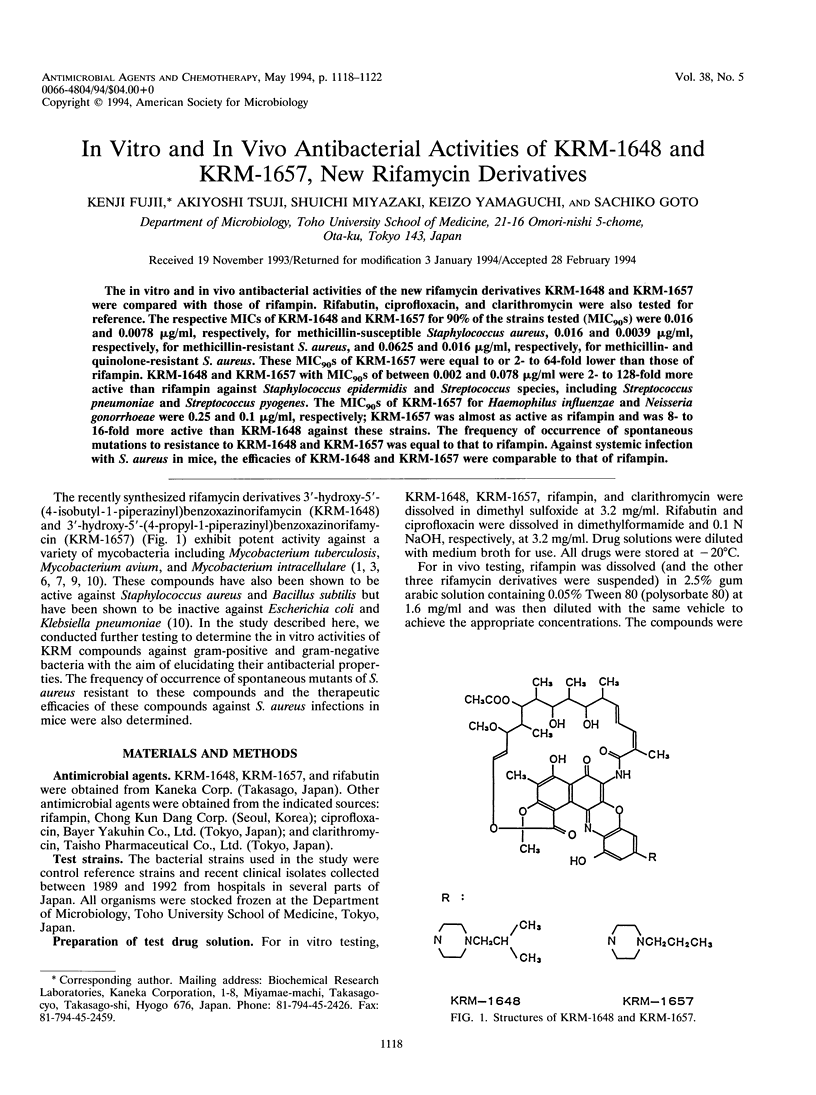

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emori M., Saito H., Sato K., Tomioka H., Setogawa T., Hidaka T. Therapeutic efficacy of the benzoxazinorifamycin KRM-1648 against experimental Mycobacterium avium infection induced in rabbits. Antimicrob Agents Chemother. 1993 Apr;37(4):722–728. doi: 10.1128/aac.37.4.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng R. H., Smith S. M., Buccini F. J., Cherubin C. E. Differences in ability of cell-wall antibiotics to suppress emergence of rifampicin resistance in Staphylococcus aureus. J Antimicrob Chemother. 1985 Feb;15(2):201–207. doi: 10.1093/jac/15.2.201. [DOI] [PubMed] [Google Scholar]
- Kuze F., Yamamoto T., Amitani R., Suzuki K. [In vivo activities of new rifamycin derivatives against mycobacteria]. Kekkaku. 1991 Jan;66(1):7–12. [PubMed] [Google Scholar]
- Saito H., Tomioka H., Sato K., Emori M., Yamane T., Yamashita K., Hosoe K., Hidaka T. In vitro antimycobacterial activities of newly synthesized benzoxazinorifamycins. Antimicrob Agents Chemother. 1991 Mar;35(3):542–547. doi: 10.1128/aac.35.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sande M. A., Scheld W. M. Combination antibiotic therapy of bacterial endocarditis. Ann Intern Med. 1980 Mar;92(3):390–395. doi: 10.7326/0003-4819-92-3-390. [DOI] [PubMed] [Google Scholar]
- Tomioka H., Saito H., Fujii K., Sato K., Hidaka T. In vitro antimicrobial activity of benzoxazinorifamycin, KRM-1648, against Mycobacterium avium complex, determined by the radiometric method. Antimicrob Agents Chemother. 1993 Jan;37(1):67–70. doi: 10.1128/aac.37.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka H., Saito H., Sato K., Yamane T., Yamashita K., Hosoe K., Fujii K., Hidaka T. Chemotherapeutic efficacy of a newly synthesized benzoxazinorifamycin, KRM-1648, against Mycobacterium avium complex infection induced in mice. Antimicrob Agents Chemother. 1992 Feb;36(2):387–393. doi: 10.1128/aac.36.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Amitani R., Kuze F., Suzuki K. [In vitro activities of new rifamycin derivatives against Mycobacterium tuberculosis and M. avium complex]. Kekkaku. 1990 Dec;65(12):805–810. [PubMed] [Google Scholar]
- Yamane T., Hashizume T., Yamashita K., Konishi E., Hosoe K., Hidaka T., Watanabe K., Kawaharada H., Yamamoto T., Kuze F. Synthesis and biological activity of 3'-hydroxy-5'-aminobenzoxazinorifamycin derivatives. Chem Pharm Bull (Tokyo) 1993 Jan;41(1):148–155. doi: 10.1248/cpb.41.148. [DOI] [PubMed] [Google Scholar]



